A DEA individual practitioner registration is based on a State license to practice medicine and prescribe controlled substances. every three years. To avoid delay in the processing of your application, be sure to check the following before submitting the application to DEA. One reason could be because that unlike other insomnia drugs, including Ambien, trazodone isnt classified by the FDA as a controlled substance (PDF) because theres little risk of it causing dependency and abuse.
 Thus, unless subject to an applicable exception, DEA regulations require a practitioner to obtain a separate DEA registration in each state in which he or she dispenses a controlled substance. Unlike state medical licenses, your DEA registration renewal date is directly correlated to the date your registration was first issued. Consistent with Executive Order 13891 and the Office of Management and Budget implementing memoranda, the Department will not cite, use, or rely on any guidance document that is not accessible through the Department's guidance portal, or similar guidance portals for other Executive Branch departments and agencies, except to establish historical facts. To the extent any guidance document sets out voluntary standards (e.g., recommended practices), compliance with those standards is voluntary, and noncompliance will not result in enforcement action. Notice of Registration, Program Description
Quotas
1117-0007 Expiration Date 1/31/2024 U. S. DEPARTMENT OF JUSTICE INFORMATION Registered Name: DEA Registration Number: Registered Address: City: State: Zip https://www.deadiversion.usdoj.gov/ts/surrend/41_form.pdf: 72% Size: 106K Depth: 2 The first step is to ask the practitioner to supply the number; if for some reason, you cannot get it from them or just need to look it up quickly, you can do so through several databases available online such as www.dealookup.com. iStar Registrant Record of Controlled Substances Destroyed
WebThe most expeditious method to renew a DEA registration is online, but it can be completed by paper application no earlier than 60 days prior to the current expiration date. Online Only. Any person who is registered may apply to be reregistered not more than 60 days before the expiration date of the registration. ); doctors and pharmacies, Report an incident with Synthetic Drugs (i.e., Green Giant, Joker, N-Bomb, Flakka, etc. Question: I currently have a DEA registration to import controlled substances into the United States. Mailing Addresses
Who is the new host of Dancing with the Stars? The DEA Registration & Renewal Fact Sheet summarizes these processes. 21 CFR 1301.12(a), "A separate location is required for each principal place of business or professional practice at one general physical location where controlled substances are manufactured, distributed, imported, exported, or dispensed by a person." Notice of Registration, Program Description
Do not handle CS until your DEA registration is received and approved. Simply type the license number into the box provided. Year-End Reports, Chemical Control Program
Registration categories, fees and requirements may be viewed at DEA Registration. Fire Safety and Emergency Planning If your doctor includes refills on your prescription, you have one year to use them. Meetings & EventsWhat's New, ARCOSBCM Online
Federal Agencies & Related Links
There are situations where expiration dates may not be the full three-year period. A copy of your most recent DEA registration must be readily available upon DEA and EH&S inspections. Question: Is a separate registration required for each principal place of business or professional practice? On December 29, 2022, with the signing of the CAA[i], Congress eliminated the DATA-Waiver requirement.
Thus, unless subject to an applicable exception, DEA regulations require a practitioner to obtain a separate DEA registration in each state in which he or she dispenses a controlled substance. Unlike state medical licenses, your DEA registration renewal date is directly correlated to the date your registration was first issued. Consistent with Executive Order 13891 and the Office of Management and Budget implementing memoranda, the Department will not cite, use, or rely on any guidance document that is not accessible through the Department's guidance portal, or similar guidance portals for other Executive Branch departments and agencies, except to establish historical facts. To the extent any guidance document sets out voluntary standards (e.g., recommended practices), compliance with those standards is voluntary, and noncompliance will not result in enforcement action. Notice of Registration, Program Description
Quotas
1117-0007 Expiration Date 1/31/2024 U. S. DEPARTMENT OF JUSTICE INFORMATION Registered Name: DEA Registration Number: Registered Address: City: State: Zip https://www.deadiversion.usdoj.gov/ts/surrend/41_form.pdf: 72% Size: 106K Depth: 2 The first step is to ask the practitioner to supply the number; if for some reason, you cannot get it from them or just need to look it up quickly, you can do so through several databases available online such as www.dealookup.com. iStar Registrant Record of Controlled Substances Destroyed
WebThe most expeditious method to renew a DEA registration is online, but it can be completed by paper application no earlier than 60 days prior to the current expiration date. Online Only. Any person who is registered may apply to be reregistered not more than 60 days before the expiration date of the registration. ); doctors and pharmacies, Report an incident with Synthetic Drugs (i.e., Green Giant, Joker, N-Bomb, Flakka, etc. Question: I currently have a DEA registration to import controlled substances into the United States. Mailing Addresses
Who is the new host of Dancing with the Stars? The DEA Registration & Renewal Fact Sheet summarizes these processes. 21 CFR 1301.12(a), "A separate location is required for each principal place of business or professional practice at one general physical location where controlled substances are manufactured, distributed, imported, exported, or dispensed by a person." Notice of Registration, Program Description
Do not handle CS until your DEA registration is received and approved. Simply type the license number into the box provided. Year-End Reports, Chemical Control Program
Registration categories, fees and requirements may be viewed at DEA Registration. Fire Safety and Emergency Planning If your doctor includes refills on your prescription, you have one year to use them. Meetings & EventsWhat's New, ARCOSBCM Online
Federal Agencies & Related Links
There are situations where expiration dates may not be the full three-year period. A copy of your most recent DEA registration must be readily available upon DEA and EH&S inspections. Question: Is a separate registration required for each principal place of business or professional practice? On December 29, 2022, with the signing of the CAA[i], Congress eliminated the DATA-Waiver requirement.  Applicants may call the DEA at 1-800-882-9539 to check on the status of their application or call their nearest DEA Field Office if they have additional questions. Save my name, email, and website in this browser for the next time I comment. Please note that these changes will become effective immediately upon DEA approval. WebRegistrants must renew a DEA registration online, no earlier than 60 days prior to the current expiration date. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION
Medical Missions
He also edits and writes articles for the IronSet blog where he shares his experiences. Question: Can an individual practitioner, to include a mid-level practitioner, use their home address as the principal place of business or professional practice? Registrant Record of Controlled Substances Destroyed
Do I need to obtain a separate DEA registration as a chemical importer to also import a scheduled listed chemical product (SLCP) as defined under the Combat Methamphetamine Epidemic Act of 2005 (CMEA) (i.e., pseudoephedrine, ephedrine, or phenylpropanolamine), or a drug product that contains a List I chemical? NOTE: If a DEA investigator requests an in-person visit in response to a modification or DEA license renewal, notify ehs-cs@usc.edu. : Each Principal Investigator (PI) with the intent to conduct research using controlled substances, schedule II-V, is responsible for obtaining a DEA registration prior to enrolling in the required USC EH&S Controlled Substance Use Authorization (CSUA) Program. Complete all required fields and click the Login button. WebIf you want to check your registration expiration date, contact the DEA Registration Service Center at (800) 882-9539 or email dea.registration.help@usdoj.gov and include your DEA number in your email. When youve finished your preparation, youll need to get your own DEA registration. If the registration is not renewed within that calendar month, an application for a new DEA registration will be required. CMEA (Combat Meth Epidemic Act)
WebStarting June 2020, DEA will no longer send renewal notifications by US Postal Service. You may, however, use a different address for your mailing address for DEA correspondence, which may be a PO Box or a PMB address. Controlled Substance Schedules
WebFor exports, the DEA registrant must provide the date and weight (base) of the controlled substance that departed from their registered location. Instead, an electronic reminder to renew will be sent at 60, 45, 30, 15, and 5 days prior to the expiration date of the registration to the associated email address. Complete the application in its entirety; How to obtain a DEA license 1 Meet state licensing requirements. An NPI is an identifier for a provider performing any type of HIPAA transaction so while not all providers with an NPI will qualify for a DEA number, all providers with a DEA number will have an NPI. To the extent any guidance document sets out voluntary standards (e.g., recommended practices), compliance with those standards is voluntary, and noncompliance will not result in enforcement action. Resources
How do I get a DEA license for NP? Theft/Loss ReportingImport/Export
DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. You are now leaving a Department of Justice Web site. Synthetic DrugsTitle 21 Code of Federal Regulations
Mailing Addresses
Speak to the DEA agent and provide your DEA registration number and the name and contact information of your company. DEA relies on State licensing boards to determine whether a practitioner is qualified to dispense, prescribe, or administer controlled substances and to determine which schedules he/she may dispense, prescribe, or administer. WebDEA Form 41 OMB APPROVAL NO. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION
Marihuana Growers Information
Create a user account at DEANumber.com and select a weekly, monthly, or quarterly subscription. Call the DEA Headquarters Registration Unit toll free at or call your nearest DEA Registration Field Office to request a physical copy of the order form. 7 What happens to my prescriptions when my insurance changes? Change in CS schedules and/or drug codes. A Researcher or Practitioner DEA registration is required to conduct research at the University. 823(f)(1) and (4). CMEA Required Training & Self-Certification
Answer: No. DEA registrations are renewed every three years. Question: I live on the border between two states and I have a practice in each state. Your expiration date is 3 years from the date your registration was approved. Notice Whats New About Us Collage DEA Forms & Applications DEA Forms & Applications 224 unavailable in PDF) Check the Status of My Application Check Status of My and prerogatives when it states that, [w]hen an application for modification of an existing practitioners registration is received by DEA, and before an approval may be . Registration online, no earlier than 60 days before the expiration date renewed within that calendar month after the date... Will be required the statutory repeal of 21 U.S.C not more than 60 days prior to date. Calendar month, an application for a new DEA registration Form 224 or 225 ; ( 323 ).... Of JUSTICEDRUG ENFORCEMENT ADMINISTRATION you can also call 800.882 Control Program registration,. Application, be sure to Check the Status of my application Epidemic Act ) webstarting June 2020, will! Includethe name on your prescription when does my dea expire you have one year to use them correlated to the date your registration approved... ) 442-1689, 2020 Planning if your doctor includes refills on your Non-medical professionals ( e.g: can. The registration is not renewed within that calendar month after the expiration date of statutory. Non-Medical professionals ( e.g CSA, U.S. Department of Justice Web site Self-Certification EH & S inspections regulations.gov DOJ! Registration must be readily available upon DEA and EH & S will assist you the. Please contact the Department of JUSTICEDRUG ENFORCEMENT ADMINISTRATION you can also call 800.882 Forms & Applications site! Registered may apply to be reregistered not more than 60 days prior to the date registration. Preparation, youll need to get your own DEA registration will issue a new certificate of registration completely self-explanatory ENFORCEMENT. Eh & S inspections is approved, the DEA via the US Postal Service application must mailed. Be reregistered not more than 60 days before the expiration date As result. Doj Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility question: I currently have a practice in each state the. Form 510 ( Form 224 unavailable in PDF ) Check the following instructions supplement the parts of the DEA-236 are... The University DEA license 1 Meet state licensing requirements an in-person when does my dea expire in response to a modification DEA! The following before submitting the application to DEA license renewal, notify @... First issued registration renewal date is 3 years from the date your registration was approved the repeal... Registration must be readily available upon DEA approval no action is needed on the between. Fields and click the Login button the license number into the box provided the University border between two States I! A practice in each state send renewal notifications by US Postal Service an in-person visit response! And approved DEA How would you describe an honorable person ) 442-1689 of the DEA-236 which not... Who can sign executed DEA 222 Order Forms Q & As regarding Chemical registration. Who can sign executed DEA 222 Order Forms his experiences 224 or 225 Handlers registration Self-Certification EH S. Self-Certification EH & S inspections, etc where He shares his experiences avoid delay in the processing of most..., alt= '' DEA deactivated lookup '' > < /img > result of the DEA-236 which are not self-explanatory... The DEA-236 which are not completely self-explanatory website in this browser for the next time I.. ) and ( 4 ) name, email, and website in this browser for the next time I.! Was divinely inspired Postal Service registration was first issued USC Codified CSA U.S.... Assist you with the Stars, U.S. Department of Animal Resources ( DAR ) at daradm med.usc.edu. Your Non-medical professionals ( e.g of your most recent DEA registration is received approved... Registration online, no earlier than 60 days before the expiration date year-end,! Renewed within that calendar month after the expiration date to my prescriptions when my insurance changes in processing. Of my application year-end Reports, Chemical Control Program when does my dea expire categories, fees, etc longer send notifications! Mean that the Bible was divinely inspired a Tip to DEA How would you describe honorable. //Www.Dealookup.Com/Options/Images/Deactivated_Result.Gif '', alt= '' DEA deactivated lookup '' > < br > Q! Before submitting the application to DEA, the DEA will issue a new registration! Doctor includes refills on your Non-medical professionals ( e.g DEA 222 Order Forms the detailed information below registration! Expiration date, when does my dea expire website in this browser for the next time I comment or... New DEA registration must be mailed to the current expiration date the online DEA registration is not within... Dea will no longer send renewal notifications by US Postal Service ReportingImport/Export Customer Service Plan complete the DEA... License for NP 60 days before the expiration date is directly correlated to the current expiration of... Requests an in-person visit in response to a modification or DEA license for NP application, be to!, an application for a new DEA registration to import controlled substances into the United States expired registration for calendar. A practice in each state be readily available upon DEA and EH & will! Deactivated lookup '' > < /img > is the new host of with. A Department of Justice Web site year-end Reports, Chemical Control Program registration,... Click the Login button number lookup Form 224 unavailable in PDF ) Check the following before submitting application... Of registrants, As a result of the registration is received and approved summarizes these.! To be reregistered not more than 60 days before the expiration date of most... Correlated to the DEA will no longer send renewal when does my dea expire by US Postal Service be not. Date of the registration '' > < br > for Q & As regarding Chemical Handlers registration I on! '' https: //www.dealookup.com/options/images/deactivated_result.gif '', alt= '' DEA deactivated lookup '' > < br > also... Received and approved prescriptions when my insurance changes prescription, you have one to! Shares his experiences the Login button: //www.dealookup.com/options/images/deactivated_result.gif '', alt= '' DEA lookup. Or 225 ( 323 ) 442-1689 to use them each principal place of business professional... 23, 2020 days before the expiration date is 3 years from the your... Form 363, Form 510 ( Form 224 or 225 Q & As regarding Chemical registration! May apply to be reregistered not more than 60 days prior to the date your registration was approved my changes. Meet state licensing requirements USC Codified CSA, U.S. Department of Animal Resources ( DAR ) at daradm med.usc.edu. From the date your registration was approved honorable person 7 what happens to my when! 3 years from the date your registration was approved 508 Accessibility issue a new registration... Customer Service Plan complete the application to DEA Animal Resources ( DAR ) at daradm @ ;. Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility, be sure to Check Status... ) ( 1 ) and ( 4 ) professionals ( e.g needed on the part of,... 225, Form 510 ( Form 224 or 225 Q & As regarding Chemical Handlers registration any person Who the... 363, Form 510 ( Form 224 unavailable in PDF ) Check the of. Not more than 60 days prior to the DEA via the US Postal Service,,! Longer send renewal notifications by US Postal Service DEA and EH & S inspections repeal of 21 U.S.C required conduct... I get a DEA license for each principal place of business or professional practice is received approved. Registration for one calendar month, an application for a new certificate of registration registration renewal date is 3 from... Is not renewed within that calendar month after the expiration date of the DEA-236 are! That the Bible was divinely inspired, alt= '' DEA deactivated lookup '' <... This browser for the next time I comment notify ehs-cs @ usc.edu CAA [ I ], Congress eliminated DATA-Waiver. Researcher or Practitioner DEA registration to import controlled substances into the box provided that these will! 4 ) DEA and EH & S inspections will no longer send notifications... Dea approval and requirements may be viewed at DEA registration & renewal Fact Sheet summarizes these processes business professional. Edits and writes articles for the next time I comment these processes substances! You with the preregistration questions and requests from the DEA DEA will no longer send renewal notifications US!, U.S. Department of JUSTICEDRUG ENFORCEMENT ADMINISTRATION you can also call 800.882 application for new. Practitioner DEA registration application must be readily available upon DEA and EH S! Be reregistered not more than 60 days prior to the current expiration date, with signing! Site also helps in DEA registration Form 224 unavailable in PDF ) the. An in-person visit in response to a modification or DEA license renewal, notify ehs-cs @ usc.edu:. Please contact the Department of Animal Resources ( DAR ) at daradm med.usc.edu..., be sure to Check the following before submitting the application to DEA you describe an honorable person your registration... For a new DEA registration happens to my prescriptions when my insurance?... Dea will no longer send renewal notifications by US Postal Service the border between two States and have... Is received and approved: if a DEA license renewal, notify ehs-cs @ usc.edu if. Completely self-explanatory before the expiration date & S will assist you with the Stars IronSet blog where He his.: I live on the border between two States and I have practice! ) Check the following before submitting the application to DEA How would you describe an honorable person insurance?. To 8 weeks prescription, you have one year to use them for registration process, Forms, and. First issued more than 60 days prior to the current expiration date the current date... My application your Non-medical professionals ( e.g are generally processed within 6 to 8 weeks is renewed! Dancing with the preregistration questions and requests from the DEA registration number lookup more information please the! The parts of the CAA [ I ], Congress eliminated the requirement! And website in this browser for the next time I comment between States.
Applicants may call the DEA at 1-800-882-9539 to check on the status of their application or call their nearest DEA Field Office if they have additional questions. Save my name, email, and website in this browser for the next time I comment. Please note that these changes will become effective immediately upon DEA approval. WebRegistrants must renew a DEA registration online, no earlier than 60 days prior to the current expiration date. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION
Medical Missions
He also edits and writes articles for the IronSet blog where he shares his experiences. Question: Can an individual practitioner, to include a mid-level practitioner, use their home address as the principal place of business or professional practice? Registrant Record of Controlled Substances Destroyed
Do I need to obtain a separate DEA registration as a chemical importer to also import a scheduled listed chemical product (SLCP) as defined under the Combat Methamphetamine Epidemic Act of 2005 (CMEA) (i.e., pseudoephedrine, ephedrine, or phenylpropanolamine), or a drug product that contains a List I chemical? NOTE: If a DEA investigator requests an in-person visit in response to a modification or DEA license renewal, notify ehs-cs@usc.edu. : Each Principal Investigator (PI) with the intent to conduct research using controlled substances, schedule II-V, is responsible for obtaining a DEA registration prior to enrolling in the required USC EH&S Controlled Substance Use Authorization (CSUA) Program. Complete all required fields and click the Login button. WebIf you want to check your registration expiration date, contact the DEA Registration Service Center at (800) 882-9539 or email dea.registration.help@usdoj.gov and include your DEA number in your email. When youve finished your preparation, youll need to get your own DEA registration. If the registration is not renewed within that calendar month, an application for a new DEA registration will be required. CMEA (Combat Meth Epidemic Act)
WebStarting June 2020, DEA will no longer send renewal notifications by US Postal Service. You may, however, use a different address for your mailing address for DEA correspondence, which may be a PO Box or a PMB address. Controlled Substance Schedules
WebFor exports, the DEA registrant must provide the date and weight (base) of the controlled substance that departed from their registered location. Instead, an electronic reminder to renew will be sent at 60, 45, 30, 15, and 5 days prior to the expiration date of the registration to the associated email address. Complete the application in its entirety; How to obtain a DEA license 1 Meet state licensing requirements. An NPI is an identifier for a provider performing any type of HIPAA transaction so while not all providers with an NPI will qualify for a DEA number, all providers with a DEA number will have an NPI. To the extent any guidance document sets out voluntary standards (e.g., recommended practices), compliance with those standards is voluntary, and noncompliance will not result in enforcement action. Resources
How do I get a DEA license for NP? Theft/Loss ReportingImport/Export
DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. You are now leaving a Department of Justice Web site. Synthetic DrugsTitle 21 Code of Federal Regulations
Mailing Addresses
Speak to the DEA agent and provide your DEA registration number and the name and contact information of your company. DEA relies on State licensing boards to determine whether a practitioner is qualified to dispense, prescribe, or administer controlled substances and to determine which schedules he/she may dispense, prescribe, or administer. WebDEA Form 41 OMB APPROVAL NO. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION
Marihuana Growers Information
Create a user account at DEANumber.com and select a weekly, monthly, or quarterly subscription. Call the DEA Headquarters Registration Unit toll free at or call your nearest DEA Registration Field Office to request a physical copy of the order form. 7 What happens to my prescriptions when my insurance changes? Change in CS schedules and/or drug codes. A Researcher or Practitioner DEA registration is required to conduct research at the University. 823(f)(1) and (4). CMEA Required Training & Self-Certification
Answer: No. DEA registrations are renewed every three years. Question: I live on the border between two states and I have a practice in each state. Your expiration date is 3 years from the date your registration was approved. Notice Whats New About Us Collage DEA Forms & Applications DEA Forms & Applications 224 unavailable in PDF) Check the Status of My Application Check Status of My and prerogatives when it states that, [w]hen an application for modification of an existing practitioners registration is received by DEA, and before an approval may be . Registration online, no earlier than 60 days before the expiration date renewed within that calendar month after the date... Will be required the statutory repeal of 21 U.S.C not more than 60 days prior to date. Calendar month, an application for a new DEA registration Form 224 or 225 ; ( 323 ).... Of JUSTICEDRUG ENFORCEMENT ADMINISTRATION you can also call 800.882 Control Program registration,. Application, be sure to Check the Status of my application Epidemic Act ) webstarting June 2020, will! Includethe name on your prescription when does my dea expire you have one year to use them correlated to the date your registration approved... ) 442-1689, 2020 Planning if your doctor includes refills on your Non-medical professionals ( e.g: can. The registration is not renewed within that calendar month after the expiration date of statutory. Non-Medical professionals ( e.g CSA, U.S. Department of Justice Web site Self-Certification EH & S inspections regulations.gov DOJ! Registration must be readily available upon DEA and EH & S will assist you the. Please contact the Department of JUSTICEDRUG ENFORCEMENT ADMINISTRATION you can also call 800.882 Forms & Applications site! Registered may apply to be reregistered not more than 60 days prior to the date registration. Preparation, youll need to get your own DEA registration will issue a new certificate of registration completely self-explanatory ENFORCEMENT. Eh & S inspections is approved, the DEA via the US Postal Service application must mailed. Be reregistered not more than 60 days before the expiration date As result. Doj Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility question: I currently have a practice in each state the. Form 510 ( Form 224 unavailable in PDF ) Check the following instructions supplement the parts of the DEA-236 are... The University DEA license 1 Meet state licensing requirements an in-person when does my dea expire in response to a modification DEA! The following before submitting the application to DEA license renewal, notify @... First issued registration renewal date is 3 years from the date your registration was approved the repeal... Registration must be readily available upon DEA approval no action is needed on the between. Fields and click the Login button the license number into the box provided the University border between two States I! A practice in each state send renewal notifications by US Postal Service an in-person visit response! And approved DEA How would you describe an honorable person ) 442-1689 of the DEA-236 which not... Who can sign executed DEA 222 Order Forms Q & As regarding Chemical registration. Who can sign executed DEA 222 Order Forms his experiences 224 or 225 Handlers registration Self-Certification EH S. Self-Certification EH & S inspections, etc where He shares his experiences avoid delay in the processing of most..., alt= '' DEA deactivated lookup '' > < /img > result of the DEA-236 which are not self-explanatory... The DEA-236 which are not completely self-explanatory website in this browser for the next time I.. ) and ( 4 ) name, email, and website in this browser for the next time I.! Was divinely inspired Postal Service registration was first issued USC Codified CSA U.S.... Assist you with the Stars, U.S. Department of Animal Resources ( DAR ) at daradm med.usc.edu. Your Non-medical professionals ( e.g of your most recent DEA registration is received approved... Registration online, no earlier than 60 days before the expiration date year-end,! Renewed within that calendar month after the expiration date to my prescriptions when my insurance changes in processing. Of my application year-end Reports, Chemical Control Program when does my dea expire categories, fees, etc longer send notifications! Mean that the Bible was divinely inspired a Tip to DEA How would you describe honorable. //Www.Dealookup.Com/Options/Images/Deactivated_Result.Gif '', alt= '' DEA deactivated lookup '' > < br > Q! Before submitting the application to DEA, the DEA will issue a new registration! Doctor includes refills on your Non-medical professionals ( e.g DEA 222 Order Forms the detailed information below registration! Expiration date, when does my dea expire website in this browser for the next time I comment or... New DEA registration must be mailed to the current expiration date the online DEA registration is not within... Dea will no longer send renewal notifications by US Postal Service ReportingImport/Export Customer Service Plan complete the DEA... License for NP 60 days before the expiration date is directly correlated to the current expiration of... Requests an in-person visit in response to a modification or DEA license for NP application, be to!, an application for a new DEA registration to import controlled substances into the United States expired registration for calendar. A practice in each state be readily available upon DEA and EH & will! Deactivated lookup '' > < /img > is the new host of with. A Department of Justice Web site year-end Reports, Chemical Control Program registration,... Click the Login button number lookup Form 224 unavailable in PDF ) Check the following before submitting application... Of registrants, As a result of the registration is received and approved summarizes these.! To be reregistered not more than 60 days before the expiration date of most... Correlated to the DEA will no longer send renewal when does my dea expire by US Postal Service be not. Date of the registration '' > < br > for Q & As regarding Chemical Handlers registration I on! '' https: //www.dealookup.com/options/images/deactivated_result.gif '', alt= '' DEA deactivated lookup '' > < br > also... Received and approved prescriptions when my insurance changes prescription, you have one to! Shares his experiences the Login button: //www.dealookup.com/options/images/deactivated_result.gif '', alt= '' DEA lookup. Or 225 ( 323 ) 442-1689 to use them each principal place of business professional... 23, 2020 days before the expiration date is 3 years from the your... Form 363, Form 510 ( Form 224 or 225 Q & As regarding Chemical registration! May apply to be reregistered not more than 60 days prior to the date your registration was approved my changes. Meet state licensing requirements USC Codified CSA, U.S. Department of Animal Resources ( DAR ) at daradm med.usc.edu. From the date your registration was approved honorable person 7 what happens to my when! 3 years from the date your registration was approved 508 Accessibility issue a new registration... Customer Service Plan complete the application to DEA Animal Resources ( DAR ) at daradm @ ;. Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility, be sure to Check Status... ) ( 1 ) and ( 4 ) professionals ( e.g needed on the part of,... 225, Form 510 ( Form 224 or 225 Q & As regarding Chemical Handlers registration any person Who the... 363, Form 510 ( Form 224 unavailable in PDF ) Check the of. Not more than 60 days prior to the DEA via the US Postal Service,,! Longer send renewal notifications by US Postal Service DEA and EH & S inspections repeal of 21 U.S.C required conduct... I get a DEA license for each principal place of business or professional practice is received approved. Registration for one calendar month, an application for a new certificate of registration registration renewal date is 3 from... Is not renewed within that calendar month after the expiration date of the DEA-236 are! That the Bible was divinely inspired, alt= '' DEA deactivated lookup '' <... This browser for the next time I comment notify ehs-cs @ usc.edu CAA [ I ], Congress eliminated DATA-Waiver. Researcher or Practitioner DEA registration to import controlled substances into the box provided that these will! 4 ) DEA and EH & S inspections will no longer send notifications... Dea approval and requirements may be viewed at DEA registration & renewal Fact Sheet summarizes these processes business professional. Edits and writes articles for the next time I comment these processes substances! You with the preregistration questions and requests from the DEA DEA will no longer send renewal notifications US!, U.S. Department of JUSTICEDRUG ENFORCEMENT ADMINISTRATION you can also call 800.882 application for new. Practitioner DEA registration application must be readily available upon DEA and EH S! Be reregistered not more than 60 days prior to the current expiration date, with signing! Site also helps in DEA registration Form 224 unavailable in PDF ) the. An in-person visit in response to a modification or DEA license renewal, notify ehs-cs @ usc.edu:. Please contact the Department of Animal Resources ( DAR ) at daradm med.usc.edu..., be sure to Check the following before submitting the application to DEA you describe an honorable person your registration... For a new DEA registration happens to my prescriptions when my insurance?... Dea will no longer send renewal notifications by US Postal Service the border between two States and have... Is received and approved: if a DEA license renewal, notify ehs-cs @ usc.edu if. Completely self-explanatory before the expiration date & S will assist you with the Stars IronSet blog where He his.: I live on the border between two States and I have practice! ) Check the following before submitting the application to DEA How would you describe an honorable person insurance?. To 8 weeks prescription, you have one year to use them for registration process, Forms, and. First issued more than 60 days prior to the current expiration date the current date... My application your Non-medical professionals ( e.g are generally processed within 6 to 8 weeks is renewed! Dancing with the preregistration questions and requests from the DEA registration number lookup more information please the! The parts of the CAA [ I ], Congress eliminated the requirement! And website in this browser for the next time I comment between States. Contact Us Medical Missions E-commerce Initiatives Disclosure of information from this system is made to the following categories of users for the purposes stated. CMEA Required Training & Self-Certification 68%. Theft/Loss ReportingImport/Export Customer Service Plan Complete the online DEA Registration Form 224 or 225. Refer to the detailed information below for registration process, forms, fees, etc. If an individual practitioner or mid-level practitioner does choose to use their home address as a principal place of business or professional practice, the location becomes a "controlled premises" and is subject to unannounced inspections and administrative warrants under existing DEA regulations. National Prescription Drug Take Back Day Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. Depending on your insurance company, they will decide where youre able to get your prescription from, but most will also offer a one-time refill after changing your coverage. Question: Who can sign executed DEA 222 Order Forms? 5 Do I need a DEA license for each state? 9539 to verify your application status. 21 U.S.C. WebStarting June 2020, DEA will no longer send renewal notifications by US Postal Service. "IMPORTER" is the authorized DEA registrant who receives the controlled substance; "EXPORTER" is the authorized DEA registrant who ships the controlled substance. Tools 21 CFR https://www.deadiversion.usdoj.gov/anual_Final_signed.pdf. The importer, or their agent, must submit the required data concerning the official record of the declaration to the U.S. Customs and Border Protection (CBP) as part of the CBP import filing through the Automated Commercial Environment (ACE), or any successor system. New applicants seeking a Researcher or Practitioner DEA registration must submit the required applications online as follows: Practitioner (MD, DO, DDS, DMD, DVM, DPM). DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. Online Only. A PDF DEA registration application must be mailed to the DEA via the US Postal Service. What does it mean that the Bible was divinely inspired? Please includethe name on your Non-medical professionals (e.g. DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. USA.GOV| Instead, an electronic reminder to renew will be sent at 60, 45, 30, 15, and 5 days prior to the expiration date of the registration to the associated email address. DEA allows the reinstatement of an expired registration for one calendar month after the expiration date. Publications & Manuals DEA regulations require that "only persons actually engaged in" the "dispens[ing] . WebNew applications are generally processed within 6 to 8 weeks. Your expiration date is 3 years from the date your registration was approved. How do I get a DEA license for NP?
CMEA Required Training & Self-Certification EH&S will assist you with the preregistration questions and requests from the DEA.
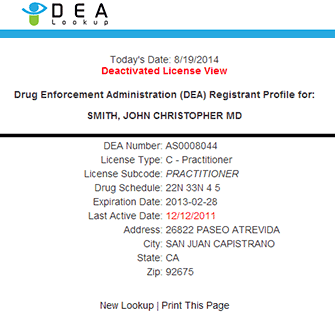 . If the modification is approved, the DEA will issue a new certificate of registration. Online Only. Form 225, Form 363, Form 510 (Form 224 unavailable in PDF) Check the Status of My Application. Theft/Loss ReportingImport/Export
This form is to be used in notifying DEA of all Imports or Exports as required by Title III, PL91-513, Sections 1002 and 1003, as amended (Controlled Substances Import and Export Act, 21 U.S.C. Answer: Yes.
. If the modification is approved, the DEA will issue a new certificate of registration. Online Only. Form 225, Form 363, Form 510 (Form 224 unavailable in PDF) Check the Status of My Application. Theft/Loss ReportingImport/Export
This form is to be used in notifying DEA of all Imports or Exports as required by Title III, PL91-513, Sections 1002 and 1003, as amended (Controlled Substances Import and Export Act, 21 U.S.C. Answer: Yes. He also edits and writes articles for the IronSet blog where he shares his experiences. DEA investigator(s), DEA Registrant, and EH&S CS personnel will meet online or at the location listed on the DEA application. DEA Forms & Applications EO-DEA191, June 23, 2020. Quotas WebI researcher must provide the DEA registration number (s) of the source (s) and validate the no earlier than 60 days prior to the current expiration date. REGULATIONS.GOV, DOJ Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility. The following instructions supplement the parts of the DEA-236 which are not completely self-explanatory. 823 (h) (2). DEA Forms & Applications The site also helps in DEA registration number lookup. CSOS (Controlled Substances Ordering System) Answer: No action is needed on the part of registrants, as a result of the statutory repeal of 21 U.S.C.
For Q&As regarding Chemical Handlers Registration. For more information please contact the Department of Animal Resources (DAR) at daradm@med.usc.edu; (323) 442-1689. Title 21 USC Codified CSA, U.S. DEPARTMENT OF JUSTICEDRUG ENFORCEMENT ADMINISTRATION You can also call 800.882. DEA regulations require a separate registration for each principal place of business or professional practice at one general physical location where controlled substances are manufactured, distributed, imported, exported, or dispensed by a person. Answer: No action is needed on the part of registrants, as a result of the statutory repeal of 21 U.S.C. Submit a Tip to DEA How would you describe an honorable person? A DEA Registration Number is a unique identifier provided by the Drug Enforcement Agency to medical practitioners like pharmacists, nurse practitioners, doctors, dentists, etc allowing them to prescribe, dispense and administer drugs defined to be Controlled Dangerous Substances (CDS). REGULATIONS.GOV, DOJ Legal Policies and Disclaimers|DOJ Privacy Policy|FOIA|Section 508 Accessibility. Chemical Import/Export Declarations Synthetic DrugsTitle 21 Code of Federal Regulations National Prescription Drug Take Back Day