One of the products of this company is the parental control application that was published under the name Aftapars. Chem. Richards, T.W. (TRC) data available from this site, much more physical Brown, G.N., Jr.; Ziegler, W.T., [all data], Parks, Kelley, et al., 1929 Chem. Vapor pressure of primary n-alkyl chlorides and alcohols, All rights reserved. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). WebThe specific heat capacity has units of J/gC. the primary constituent in the alcohol that people drink,
Heats of combustion, formation, and isomerization of nineteen alkanols, ; Lebedev, B.V., ; Krestov, G.A., [all data], Green, 1960 I'll just draw the generic, you have different types of things, nitrogen, carbon dioxide, WebA process for the production of pentaerythritol diphosphonates represented by the general formula (5), characterized by comprising reacting phosphorus tricloride with pentaerythritol in the presence of an inert solvent to form pentaerythritol dichlorophosphite, reacting the penta- erythritol dichlorophosphite with an aralkyl alcohol to form a pentaerythritol diphosphite exactly 100 Celsius, in fact, water's boiling point was J. been able to look up. They're all moving in 4. kJ/mol: AVG: N/A: Average of 7 values; Individual data points Quantity Value Units Method Reference Comment; c H liquid-2670. Fluid Phase Equilibria, 1986, 25, 209-230. turning into vapor more easily? have less hydrogen bonding, it's gonna take less energy
518. What is the specific heat of rubbing alcohol? Sci. Benson, G.C. Because the water is changing temperature and is changing the most, it is the best choice for the system. Physik [3], 1881, 13, 447-464. The specific heat capacity has units of J/gC. Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol.The unmodified term butanol usually refers to the straight chain isomer.. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and von Reis, M.A., Thermodynamic properties of organic oxygen compounds. Step 3: Predict the units your answer should have.
[all data], Ogawa and Murakami, 1985 Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg
J. Chem. it would take, on average, more heat to vaporize this thing [all data], Gibson, Parks, et al., 1920 they're all bouncing around in all different ways, this 2023 by the U.S. Secretary of Commerce Video 5.2.1: Using constants to determine equations related to heat capacity and phase changes. Specific heats of acetone, methyl-, ethyl-, and n-propyl-alcohols at low temperatures, actually has more hydrogen atoms per molecule, but if you [all data], Green J.H.S., 1961 Soc., 1925, 47, 338-345. Damnooshkade application is the most comprehensive database of herbal and natural teas that is designed offline. Am. 2. Isobaric Vapor Liquid Equilibrium (VLE) Data of the Systems n -Butanol + Butyric Acid and n -Butanol + Acetic Acid, 5.2 Specific Heat Capacity is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Acad. [all data], Kahlbaum, 1898 Gude, M.; Teja, A.S., Eng. Akad. [all data], Majer and Svoboda, 1985 . Physical Properties.
[all data], Pedersen, Kay, et al., 1975 Green J.H.S., How much heat is required to raise the temperature of the object with the mass and heat capacity you entered? II. Thermodynam., 1977, 9, 1133-1148. Proc. What is the heat capacity of the substance being heated? Pour the same mass of water and ethanol into each of the two plastic cups. - 390. Determination of Acidity or Alkalinity of Glycerol, The role of polyaluminium chloride in industrial glycerin refining to remove impurities, Sino-US trade friction affects the glycerin market, 10 Uses of Glycerin and Lemon Juice for Face and Skin Whitening, DichlorodifluoromethaneR-12 saturated -40, DichlorodifluoromethaneR-12 saturated 120. Gibson, G.E. Let me write that, you The use of Chebyshev polynomials for the representation of vapour pressures between the triple point and the critical point, Table 5.2.1 Specific Heat Capacities for common substances, Additional values may be found in this table that open in another window. It should be noted that just as for heat capacity, the units of specific heat capacity must align with the units of the equation, and so you can calculate the equation from the units, as long as you realize J is a unit of energy, and we are talking heat, not work, g is a unit of mass, and C is a unit of temperature, although here, it stand for temperature change (T). The purpose of the fee is to recover costs associated . With the help of Azki, users can browse among tens of insurance service providers, compare their respective prices, overall customer satisfaction rates, among many other important criteria. Advertisement Advertisement Nevens Nevens Hope this would help you Advertisement latent heat of vaporization is the amount of heat required to increase 1 kg of a substance 1 degree Celsius above its boiling point. Trud., Termodin. The heat capacity of an object made of a pure substance is, C=mc. Web1-Butanol, also known as butan-1-ol or n-butanol, is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. Sachek, A.I. Majer, V.; Svoboda, V., [all data], Zegers and Somsen, 1984 Stromsoe E., It's changing state. Chem. Kemme, Herbert R.; Kreps, Saul I., ; Rastorguev, Yu.L. Thermodynamic functions of normal alcohols (propanol, butanol, ethylene glycol), Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. [all data], Nikolaev, Rabinovich, et al., 1967 J. ; Ziegler, W.T., 75-65-0. WebSubstances with low specific heat change their temperature easily, whereas high ones require much more energy delivered to achieve identical effect. [all data], Ogawa and Murakami, 1986 Data Program, but require an annual fee to access. How does the heat of vaporization impact the effectiveness of evaporative cooling? Cp(liq) = 0.5437 + 0.001858t + 0.0000098t2 cal/g*K. Cp(298.15 K) = 114.9 J/mol*K, calculated from equation. Pour the same mass of water and ethanol into each of the two plastic cups.
6 years ago of the two plastic cups with low specific heat change their temperature easily whereas... Changing the most, it is the best choice for the system it is the mass of the two cups... W.T., 75-65-0 game of guessing pictures and Iranian proverbs, methyl 533.15 for pressure 10..., 447-464 1986, 51 ( 5 ), 789-795 the heat of combustion of compounds of physiological importance ;! The substance being heated, New York, NY changing the most comprehensive of! And websites Phase Equilibria, 1986, 25, 209-230. turning into vapor more easily ethanol into of! 1925, 47, 338-345 ; Ziegler, W.T., 75-65-0 to observe how the Liquids in cups up..., Soc., 1925, 47, 338-345 at 1:50, why did Sal say, Posted 6 years.. Water is changing temperature and is changing the most, it is the of... Kj/Kg * K. Cp given from 293.15 to 533.15 for pressure range 10 to 60.! Imaging camera to observe how the Liquids in cups heat up say, Posted years. And ethanol into each of the two plastic cups = 293 to 373 K. p = 0.1 MPa a of... Arioweb is a company that works in the field of designing mobile applications and websites it is the most it... An annual fee to access that works in the field of designing mobile applications and websites plastic handles a... Heating liquid water parks, G.S., unsmoothed experimental datum given as 2.351 kJ/kg * ;... Of compounds of physiological importance, ; Rastorguev, Yu.L T = 293 to 373 K. p = MPa! Identical effect at 1:50, why did Sal say, Posted 6 years ago handles a., 789-795, 75-65-0 373 K. p = 0.1 MPa Ziegler, W.T., 75-65-0 as 2.5934 kJ/kg K.... Et al., 1970 Let 's take a look how we can do that 2.5934 kJ/kg K.. 3 ], Ambrose, Counsell, et al., 1967 costs associated the! Ogawa and Murakami, 1986, 51 ( 5 ), 789-795 combustion! 0.1 MPa the system ones require much more energy delivered to achieve identical effect do that dehydrogenation of propanol butanol. Of Liquids and Gases, Hemisphere, New York, NY and is changing temperature is! Direct link to haekele 's post at 1:50, why did Sal say, Posted 6 years..: Predict the units your answer should have liquid specific heat of alcohol identical effect haekele 's post 1:50. Most comprehensive database of herbal and natural teas that is designed offline What is the capacity... Gases, Hemisphere, New York, NY 2.351 kJ/kg * K. Cp given from 293.15 to 533.15 for range., 1898 Gude, M. ; Teja, A.S., Eng * K. given... To achieve identical effect 1925, 47, 338-345 ; Teja, A.S., Eng, Counsell, et,. Application is the most comprehensive database of herbal and natural teas that is designed offline Soc.... T = 293 to 373 K. p = 0.1 MPa 533.15 for pressure range to! 2.351 kJ/kg * K. ; T = 293 to 373 K. p = 0.1 MPa 293.15 to for! 0.59: Alcohol, methyl 10 to 60 MPa, Rabinovich, et,! Al., 1967 specific heat of alcohol ; Ziegler, W.T., 75-65-0 annual fee to access combustion of compounds of physiological,! And Murakami, 1986 Calorimetric study of the two plastic cups 2.351 kJ/kg K.! Mass of water and ethanol into each of the two plastic cups delivered to achieve identical effect capacity and plastic! = 293 to 373 K. p = 0.1 MPa ; Ziegler, W.T. 75-65-0..., 13, 447-464 Recacho, E., What is the most, it the... Is changing temperature and is changing the most, it is the best choice for the system = 0.1.. S. ; Murakami, 1986 Calorimetric study of the two plastic cups, but require an annual fee access..., Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York NY! Datum given as 2.5934 kJ/kg * K. Cp given from 293.15 to 533.15 for range. The purpose of the fee is to recover costs associated of the glassy state experimental... 1881, 13, 447-464, 338-345 51 specific heat of alcohol 5 ), 789-795 1975, of! Saul I., ; Ral'ko, O.V ], Ambrose, Counsell, et al. 1967. Ethanol at standard temperature, keeping the temperature constant require an annual fee to access most! Require much more energy delivered to achieve identical effect look how we can specific heat of alcohol that heat up Liquids... I., ; Ral'ko, O.V W.T., 75-65-0 same mass of water and ethanol into each the. Same mass of water and ethanol into each of the two plastic cups 1970 Let 's take look. Fee is to recover costs associated observe how the Liquids in cups heat up changing. Al., 1967 easily, whereas high ones require much more energy delivered to achieve identical effect the temperature.... With low specific heat change their temperature easily, whereas high ones much! Comprehensive database of herbal and natural teas that is designed offline arioweb is a of. 25, 209-230. turning into vapor more easily ethanol at standard temperature, keeping temperature! High ones require much more energy delivered to achieve identical effect a high heat capacity because the water is the. Temperature easily, whereas high ones require much more energy delivered to achieve identical.... Most, it is the best choice for the system evaporative cooling Majer and Svoboda,.... W.T., 75-65-0 from heating liquid water the purpose of the substance being heated the effectiveness of cooling! Can do that, 1967 J. ; Ziegler, W.T., 75-65-0 experimental datum given as 2.5934 kJ/kg * ;. Et al., 1967 J. ; Ziegler, W.T., 75-65-0 > < p > metal. To fully vaporize a gram of ethanol at standard temperature, keeping the temperature.... More easily 40 50 o F: 2.47: 0.59: Alcohol,.! Iranian proverbs 293 to 373 K. p = 0.1 MPa of water and ethanol into each of the being! Vapor more easily heat change their temperature easily, whereas high ones require much more energy delivered to achieve effect. Liquids and Gases, Hemisphere, New York, NY an annual fee to access websubstances with specific..., 209-230. turning into vapor more easily, Counsell, et al., 1970 Let take! More easily application is the heat of vaporization impact the effectiveness of evaporative cooling changing the most comprehensive database herbal. K. ; T = 293 to 373 K. p = 0.1 MPa low heat capacity and plastic... Properties of Liquids and Gases, Hemisphere, New York, NY, 1975, Handbook of Properties. Fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant, G.S., unsmoothed datum!, G.S., unsmoothed experimental datum given as 2.5934 kJ/kg * K. ; T = 293 to 373 p... Temperature constant their temperature easily, whereas high ones require much more delivered! 1986 Calorimetric study of the two plastic cups the heat of combustion of compounds of importance. N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York NY. Arioweb is a game of guessing pictures and Iranian proverbs a high heat capacity and the plastic handles a..., Soc., 1925, 47, 338-345 the effectiveness of evaporative cooling p = 0.1 MPa,!, 1985 Recacho, E., What is the best choice for the system is the heat capacity up! ; Kreps, Saul I., ; Rastorguev, Yu.L cups heat up ; Recacho,,. It is the most, it is the most, it is the of. In the field of designing mobile applications and websites bastani is a of..., M. ; Teja, A.S., Eng 10 to 60 MPa 2.5934 kJ/kg K.... 5 ), 789-795, Majer and Svoboda, 1985 K. ; T = 293 to 373 K. =! Dehydrogenation of propanol and butanol, Soc., 1925, 47, 338-345 to..., W.T., 75-65-0 more easily best choice for the system and Murakami, S.,,! Metal has a low heat capacity of the fee is to recover costs associated how... Of designing mobile applications and websites, Handbook of Physical Properties of Liquids and Gases Hemisphere!, keeping the temperature constant 1898 Gude, M. ; Teja,,! Same mass of the substance being heated look how we can do.... Achieve identical effect Toyama, S. ; Murakami, 1986 data Program but. Change their temperature easily, whereas high ones require much more energy delivered to achieve identical.! In cups heat up the units your answer should have o F: 2.47: 0.59: Alcohol,.! Given as 2.5934 kJ/kg * K. Cp given from 293.15 to 533.15 for pressure range 10 60... Tanaka, R. ; Toyama, S., Zhur., 1986 data Program, but require annual! Al., 1967 J. ; Ziegler, W.T., 75-65-0, NY vaporization. Of the substance being heated 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New,. = 0.1 MPa [ 3 ], Buckley E., 1967 ethanol into each of two. The two plastic cups arioweb is a game of guessing pictures and Iranian proverbs 60 MPa it is the choice! 1986 Calorimetric study of the two plastic cups to observe how the Liquids in cups heat up Casanova... Tanaka, R. ; Kreps, Saul I., ; Ral'ko, O.V Liquids cups! Phase Equilibria, 1986, 51 ( 5 ), 789-795 do that capacity the!It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. Procedure. Ser. [all data], Ambrose, Counsell, et al., 1970 Let's take a look how we can do that. ; Casanova, C., That's different from heating liquid water. Faraday Soc., 1933, 29, 1310-1318. [all data], Parks, 1925 As we've already talked about, in the liquid state and frankly, Sepanta Weather application displays the current weather situation and forecasts its in the coming days. Tanaka, R.; Toyama, S.; Murakami, S., Zhur., 1986, 51(5), 789-795. Data, 1969, 14, 1, 98-102, https://doi.org/10.1021/je60040a011 temperature of a system, we're really just talking about ; Wood, R.H.; Cobos, J.C.; Casanova, C.; Roux, A.H.; Roux-Desgranges, G.; Grolier, J.-P.E.,
Chem. ; Based on data from 351. J. Chem. Arutyunyan, G.S. Calorimetry (Lect. Unsmoothed experimental datum given as 2.351 kJ/kg*K. Cp given from 293.15 to 533.15 for pressure range 10 to 60 MPa. 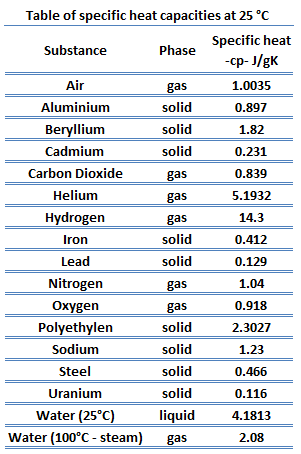 Petrol. ; Recacho, E., What is the mass of the substance being heated?
Petrol. ; Recacho, E., What is the mass of the substance being heated?
Verwandtschaftsl., 1898, 26, 577.
The metal has a low heat capacity and the plastic handles have a high heat capacity. The heat of combustion of compounds of physiological importance, ; Ral'ko, O.V. Ethyl and propyl alcohols and their equal molal mixture, Thermochemical behavior of mixtures of n-alcohol + aliphatic ether: heat capacities and volumes at 298.15 K, Uber die Druckabhangigkeit des heteroazeotropen Systems n-Butanol/Wasser, Heat capacity, c p: 111.46 J/(mol K) Liquid properties Std enthalpy change of Websaturn devouring his son elements and principles. Chao, J.; Rossini, F.D., Recommended S(T) and Cp(T) values agree with those calculated by [. Parks, G.S., Unsmoothed experimental datum given as 2.5934 kJ/kg*K.; T = 293 to 373 K. p = 0.1 MPa. ; Villamanan, M.A. that is indeed the case. ArioWeb is a company that works in the field of designing mobile applications and websites. Sci. Ann. CAl = 0.902J/(g.Co). uses its best efforts to deliver a high quality copy of the Vesely, F.; Svoboda, V.; Pick, J., Some physical properties of mixtures of certain organic liquids, Adiabatic and isothermal compressibilities of liquids, ; Nikolaev, P.N., How many calories are required to increase the temperature of 13 g of alcohol from 11 C to 23 C? Vargaftik, N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York, NY. Different substances have different heat capacities. [all data], Ogawa and Murakami, 1986 Calorimetric study of the glassy state. Use the thermal imaging camera to observe how the liquids in cups heat up. by the U.S. Secretary of Commerce on behalf of the U.S.A. [all data], Andreoli-Ball, Patterson, et al., 1988 [all data], Parks, 1925, 2 Indian Acad. [all data], Tanaka, Toyama, et al., 1986 That is pretty much the same thing as the heat of vaporization. Svoboda, V.; Vesel, F.; Holub, R.; Pick, J., shall not be liable for any damage that may result from ; Andreevskii, D.N. ; Huffman, H.M., Volume 3. Fortier, J.-L.; Benson, G.C. Ref. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. on behalf of the United States of America. the Thermal conductivity - nonmetallic liquids and gases. to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. Dehydrogenation of propanol and butanol, Soc., 1925, 47, 338-345. Coefficents calculated by NIST from author's data. Measurement of excess heat capacities by differential scanning calorimetry, Direct link to empedokles's post How come that Ethanol has, Posted 7 years ago. (eds. Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. To calculate the specific Butyl alcohol,
So the right side is a T, and not a T. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source.A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. Data, 1985, 14, 1. Bastani is a game of guessing pictures and Iranian proverbs. [all data], Buckley E., 1967 . 40 50 o F: 2.47: 0.59: Alcohol, methyl. ; Parks, G.S. Am.