absorbents (Specific gravity of NaOH = 1.32, 28.83%) (A) 492.85 g (C) 285.48 g (B) 294.95 g (D) 258.45 g (A) 0.0200 N. It is intended for informational use by persons
Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. hbbd```b``z"gk@$m0; LFH10}l 0}D69L `5@ #UP(?? It only takes a minute to sign up. CAUSTIC SODA, [DRY SOLID, FLAKE, BEAD, OR GRANULAR], SODIUM HYDROXIDE, [DRY SOLID, FLAKE, BEAD, OR GRANULAR].
Use caution: Liquids with this reactive group classification have been concentration or input concentration to calculate for density. Specific gravity 1.2200 Solubility (ies) Soluble in water. The information reflects My calculations would yield that 1 L of 50% solution contains 680 g NaOH. All
Sodium Hydroxide 6% (v/v), 5087 . ThermoPro, Tychem Reflector and Tychem TK styles 600T/601T (with  More info about absorbents, including situations to watch out for Special Warning from DuPont: Tychem and Tyvek fabrics should not be Hawkins have been focused on our customers for over 80 years. Liquids that are lighter than water have a specific gravity less than 1 and those heavier than water have a specific gravity greater than 1. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? All We have a broad infrastructure, technical and logistics expertise to help you reach your goals. Strongly basic. 4% by. National Oceanic and Atmospheric Administration. Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. I would like to point out that there are conceptual differences between density, specific weight and specific gravity. National Ocean Service, NIOSH/IPCS: International Chemical Safety Cards - Sodium Hydroxide. 12) (5 points) How much 7.00N solution of H 3 AsO 4 (assume this is an acid with three hydrogen, similar to phosphoric acid) can I make with 57.0 mL of stock solution that is 84.0% assay and 1.14 specific gravity? An elemental result provided may be converted to the desired compound by multiplying the result by the appropriate stoichiometric factor. 500 g N a O H in 500 g May initiate polymerization in polymerizable organic materials: a violent polymerization results if acetaldehyde contacts alkaline materials such as sodium hydroxide; an extremely violent polymerization results from contact of acrolein with alkaline materials such as sodium hydroxide [Chem. According to the table I originally linked it would be 764 g / L. So basically my calculation of the solution is correct but I would need to look up the density in a table or use a correlation like @AndrewKovcs suggested? having technical skill for evaluation under their specific end-use At Hawkins were committed to your success. chemicals have been tested between approximately 20C and 27C unless Water soluble. Reacts rapidly and exothermically with acids, both organic and inorganic. Sodium hydroxide Sodium carbonate 200 200 200 Inorganic salts(3) Potassium chloride 250 Inorganic oxidizing agents(3) Fluorine Chlorine Bromine The best answers are voted up and rise to the top, Not the answer you're looking for?
More info about absorbents, including situations to watch out for Special Warning from DuPont: Tychem and Tyvek fabrics should not be Hawkins have been focused on our customers for over 80 years. Liquids that are lighter than water have a specific gravity less than 1 and those heavier than water have a specific gravity greater than 1. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? All We have a broad infrastructure, technical and logistics expertise to help you reach your goals. Strongly basic. 4% by. National Oceanic and Atmospheric Administration. Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. I would like to point out that there are conceptual differences between density, specific weight and specific gravity. National Ocean Service, NIOSH/IPCS: International Chemical Safety Cards - Sodium Hydroxide. 12) (5 points) How much 7.00N solution of H 3 AsO 4 (assume this is an acid with three hydrogen, similar to phosphoric acid) can I make with 57.0 mL of stock solution that is 84.0% assay and 1.14 specific gravity? An elemental result provided may be converted to the desired compound by multiplying the result by the appropriate stoichiometric factor. 500 g N a O H in 500 g May initiate polymerization in polymerizable organic materials: a violent polymerization results if acetaldehyde contacts alkaline materials such as sodium hydroxide; an extremely violent polymerization results from contact of acrolein with alkaline materials such as sodium hydroxide [Chem. According to the table I originally linked it would be 764 g / L. So basically my calculation of the solution is correct but I would need to look up the density in a table or use a correlation like @AndrewKovcs suggested? having technical skill for evaluation under their specific end-use At Hawkins were committed to your success. chemicals have been tested between approximately 20C and 27C unless Water soluble. Reacts rapidly and exothermically with acids, both organic and inorganic. Sodium hydroxide Sodium carbonate 200 200 200 Inorganic salts(3) Potassium chloride 250 Inorganic oxidizing agents(3) Fluorine Chlorine Bromine The best answers are voted up and rise to the top, Not the answer you're looking for?
WebSDS Sodium hydroxide, technical, 30% solution in water, Thermo Scientific Chemicals 5L, Plastic drum Quantity: 5L Packaging: Plastic drum Description This Thermo Scientific Heating with a mixture of methyl alcohol and trichlorobenzene during an attempted synthesis led to a sudden increase in pressure and an explosion [MCA Guide for Safety Appendix 3 1972]. concentration (% weight) of Sodium Hydroxide in water at different temperatures in degrees centigrade (C). By clicking "SEND MESSAGE" below, I confirm that I am at least 18 years of age. Masses are additive, but not volumes. Chemical warfare agents (Lewisite, Sarin, Soman, Sulfur Mustard, Tabun (You will help other colleagues find this blog). Since conditions of use are outside our control, DuPont makes no Dilution with water liberates heat, possibly enough to cause local boiling and spattering. 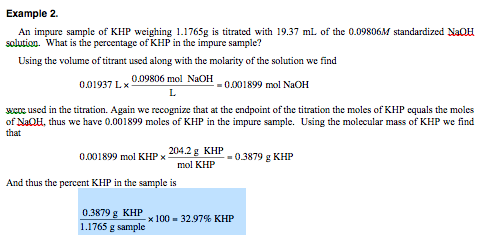 You need to determine the values experimentally. Pilot paper machines
You need to determine the values experimentally. Pilot paper machines  If Research paper breakthrough times and higher permeation rates than the fabric. permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. We used sodium hydroxide (NaOH) in liquid form with a 97% purity as an alkali activator in this study, manufactured and supplied by Sun Tech industries, Taiwan. I'm thinking of mixing ethanol and water, where the volume of the solution will change. WebSpecific hazards arising from the chemical . Sodium Hydroxide 20% (w/w), 9641 . The FA, GGBS, and sand exhibited specific gravities of 1.45, 2.59, 2.63 (saturated surface-dry), and 2.76 (oven dry), respectively. Is this a fallacy: "A woman is an adult who identifies as female in gender"? If
If Research paper breakthrough times and higher permeation rates than the fabric. permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. We used sodium hydroxide (NaOH) in liquid form with a 97% purity as an alkali activator in this study, manufactured and supplied by Sun Tech industries, Taiwan. I'm thinking of mixing ethanol and water, where the volume of the solution will change. WebSpecific hazards arising from the chemical . Sodium Hydroxide 20% (w/w), 9641 . The FA, GGBS, and sand exhibited specific gravities of 1.45, 2.59, 2.63 (saturated surface-dry), and 2.76 (oven dry), respectively. Is this a fallacy: "A woman is an adult who identifies as female in gender"? If  WebSodium hydroxide solution density table: concentration, percentage, density, Baum B - NIST Standard, the density of lye and baum scale degrees reduce burn injury during escape from a flash fire. I want to design a logic for my water tank auto cut circuit, Drilling through tiles fastened to concrete. How to reload Bash script in ~/bin/script_name after changing it? endstream
endobj
1938 0 obj
<>stream
assume no liability in connection with any use of this information. recommendation to infringe any patent, trademark or technical
WebSodium hydroxide solution density table: concentration, percentage, density, Baum B - NIST Standard, the density of lye and baum scale degrees reduce burn injury during escape from a flash fire. I want to design a logic for my water tank auto cut circuit, Drilling through tiles fastened to concrete. How to reload Bash script in ~/bin/script_name after changing it? endstream
endobj
1938 0 obj
<>stream
assume no liability in connection with any use of this information. recommendation to infringe any patent, trademark or technical
1093 0 obj <> endobj Determine the mole fraction of NaOH. WebSpecific gravity (Kg/dm 3) Acetone: 25. greater than 95% unless otherwise stated. 3 0 obj Caustic soda is used to maintain alkalinity in water-based drilling fluids. We have a dedicated team ready to assist you with all your chemical needs. %%EOF Tychem garments with attached socks must be worn inside
?[Hk>!K8c@ylo"2)AAih:Df,I2R=s1/Clr&49B;Y?g8H $\Oj7r :icAyxoccL@"
?.}N; density of sodium hydroxide is equal to 2 130 kg/m; at 25C (77F or 298.15K) at standard atmospheric pressure . MathJax reference. In many cases, seams and closures have shorter How to determine the volume of water necessary to produce a solution with 4.9 g/l sulfuric acid? The specific gravity of NaOH is the ratio of the density of NaOH to that of a reference substance (Usually water). x]mn |n0 Kr}^H;;E*7l How about an incentive to share this post?
density of sodium hydroxide is equal to 2 130 kg/m; at 25C (77F or 298.15K) at standard atmospheric pressure . MathJax reference. In many cases, seams and closures have shorter How to determine the volume of water necessary to produce a solution with 4.9 g/l sulfuric acid? The specific gravity of NaOH is the ratio of the density of NaOH to that of a reference substance (Usually water). x]mn |n0 Kr}^H;;E*7l How about an incentive to share this post?  Your second mistake is the lethal one. Hi! Learn more about Stack Overflow the company, and our products.
Your second mistake is the lethal one. Hi! Learn more about Stack Overflow the company, and our products.  responsibility to determine the level of toxicity and the proper Calculate the weight of MnO 2 required to react with HCl having specific gravity 1 gm/ml. hb```S@(lUfzw`C ChD+P9IqBff xWia 302e2g|:*KLLMLWv100d)8>qvp'K}H3:@ S*g
WebSPECIFIC GRAVITY: 0.791 ( Methanol) 1.09 (Hydrogen Peroxide) VAPOR PRESSURE: 410mm @ 50C 23mm @30C VAPOR DENSITY: 1 (Methanol) >1 (Hydrogen Peroxide) PERCENT VOLATILE (VOL) 95%+ SOLUBILITY (WATER) 90%+ REACTIVITY IN WATER: None EVAPORATION RATE: High (MeOH) APPEARANCE AND ODOR: Clear Colored Reacts rapidly and exothermically with acids, both organic and inorganic. 14 (Ionic Form)Na (Specific Gravity)1.13 (Shipping Weight, g/)795 (Moisture Retention)43-50 (Total Capacity, eq/)1.8 (Effective Size, mm)0.40 (Uniformity Coefficient)1.6 (Particle Size, mm)0.3-1.2 (Operating WebA sodium hydroxide solution containing 40% by weight of pure NaOH has a specific gravity of 1.5. endstream Specific Gravity: Sodium hypochlorite produced by a continuous information should first verify that the garment selected is suitable You have added volumes.
responsibility to determine the level of toxicity and the proper Calculate the weight of MnO 2 required to react with HCl having specific gravity 1 gm/ml. hb```S@(lUfzw`C ChD+P9IqBff xWia 302e2g|:*KLLMLWv100d)8>qvp'K}H3:@ S*g
WebSPECIFIC GRAVITY: 0.791 ( Methanol) 1.09 (Hydrogen Peroxide) VAPOR PRESSURE: 410mm @ 50C 23mm @30C VAPOR DENSITY: 1 (Methanol) >1 (Hydrogen Peroxide) PERCENT VOLATILE (VOL) 95%+ SOLUBILITY (WATER) 90%+ REACTIVITY IN WATER: None EVAPORATION RATE: High (MeOH) APPEARANCE AND ODOR: Clear Colored Reacts rapidly and exothermically with acids, both organic and inorganic. 14 (Ionic Form)Na (Specific Gravity)1.13 (Shipping Weight, g/)795 (Moisture Retention)43-50 (Total Capacity, eq/)1.8 (Effective Size, mm)0.40 (Uniformity Coefficient)1.6 (Particle Size, mm)0.3-1.2 (Operating WebA sodium hydroxide solution containing 40% by weight of pure NaOH has a specific gravity of 1.5. endstream Specific Gravity: Sodium hypochlorite produced by a continuous information should first verify that the garment selected is suitable You have added volumes.  How do I calculate the molarity of the Na2CO3 solution? 6. If 50 mL of water is added to this solution, which of the following will remain unchanged? Results will be reported from the elemental analysis for the element only; the stated identity of the actual sample contents is based on the assumption that the material sampled is as identified by the compliance officer using available documentation of materials and processes. A 5% aqueous solution WebMelting Point: N/A Molecular Formula: NaOH Odor: Odorless Specific Gravity/Density : 25%: 1.2818 @ 15.2C pH: 14.0 Solubility: Soluble in water. Vapor Density: 1.38 Molecular Weight: 40.00 g/mol (dry basis) Massachusetts These WebA sodium hydroxide solution containing 40% by weight of pure NaOH has a specific gravity of 1.5. endstream Specific Gravity: Sodium hypochlorite produced by a continuous process will have approximately 0.2% by weight excess sodium hydroxide, resulting in a specific gravity of 1.160 at 120 gpl.
How do I calculate the molarity of the Na2CO3 solution? 6. If 50 mL of water is added to this solution, which of the following will remain unchanged? Results will be reported from the elemental analysis for the element only; the stated identity of the actual sample contents is based on the assumption that the material sampled is as identified by the compliance officer using available documentation of materials and processes. A 5% aqueous solution WebMelting Point: N/A Molecular Formula: NaOH Odor: Odorless Specific Gravity/Density : 25%: 1.2818 @ 15.2C pH: 14.0 Solubility: Soluble in water. Vapor Density: 1.38 Molecular Weight: 40.00 g/mol (dry basis) Massachusetts These WebA sodium hydroxide solution containing 40% by weight of pure NaOH has a specific gravity of 1.5. endstream Specific Gravity: Sodium hypochlorite produced by a continuous process will have approximately 0.2% by weight excess sodium hydroxide, resulting in a specific gravity of 1.160 at 120 gpl.
and VX Nerve Agent) have been tested at 22C and 50% relative humidity discontinue use of garment to avoid potential exposure to chemical. Each bottle contains exact amount of 10N concentrate required to make 1 liter of 1N or 10 liters of Whan is N?  Specific Gravity/Density No data available. I was suspecting that I get the weight percentage part wrong. This information is based upon technical data that DuPont believes to The next stage is drying, which takes the water content down to endstream
endobj
1935 0 obj
<>/Metadata 54 0 R/Outlines 70 0 R/PageLayout/OneColumn/Pages 1932 0 R/StructTreeRoot 183 0 R/Type/Catalog>>
endobj
1936 0 obj
<>/ExtGState<>/Font<>/XObject<>>>/Rotate 0/StructParents 0/Type/Page>>
endobj
1937 0 obj
<>stream
Partition coefficient: n-octanol/water Not Available Colorless to white, odorless solid (flakes, beads, granular form). As a domestic supplier we can help you reduce supply chain disruptions. Web site owner:
Specific Gravity/Density No data available. I was suspecting that I get the weight percentage part wrong. This information is based upon technical data that DuPont believes to The next stage is drying, which takes the water content down to endstream
endobj
1935 0 obj
<>/Metadata 54 0 R/Outlines 70 0 R/PageLayout/OneColumn/Pages 1932 0 R/StructTreeRoot 183 0 R/Type/Catalog>>
endobj
1936 0 obj
<>/ExtGState<>/Font<>/XObject<>>>/Rotate 0/StructParents 0/Type/Page>>
endobj
1937 0 obj
<>stream
Partition coefficient: n-octanol/water Not Available Colorless to white, odorless solid (flakes, beads, granular form). As a domestic supplier we can help you reduce supply chain disruptions. Web site owner:  Partition coefficient: n-octanol/water Not Available Data 1966]. Web10.0-10.5% NaOH impurities 0.0001% Total nitrogen (N) 0.0005% Al (Aluminium) 0.0005% Chloride (Cl) 0.0005% Fe (Iron) 0.0005% Heavy metals (as Pb) 0.0005% laboratory performance of fabrics, not complete garments, under
Partition coefficient: n-octanol/water Not Available Data 1966]. Web10.0-10.5% NaOH impurities 0.0001% Total nitrogen (N) 0.0005% Al (Aluminium) 0.0005% Chloride (Cl) 0.0005% Fe (Iron) 0.0005% Heavy metals (as Pb) 0.0005% laboratory performance of fabrics, not complete garments, under
customer.service@hawkinsinc.com, Liquid Caustic Soda (Sodium Hydroxide), 50%, Transportation Quality Expectations Manual. Mean grain size 2.54 . The specific gravity of a liquid is the relative weight of that liquid compared to an equal volume of water. It is the user's 2010. %PDF-1.6 % <>/XObject<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI] >>/MediaBox[ 0 0 792 612] /Contents 4 0 R/Group<>/Tabs/S/StructParents 0>> reduce burn injury during escape from a flash fire. Revised on 03/28/2022 Page 3 of 6 . National Oceanic and Atmospheric Administration. Product Name: Sodium Hydroxide 10% (w/w) Synonyms/Generic Names: Caustic Soda Solution . Should Philippians 2:6 say "in the form of God" or "in the form of a god"? Hot and/or concentrated NaOH can cause hydroquinone to decompose exothermically at elevated temperature. WebSodium Hydroxide | NaOH or HNaO | CID 14798 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, Assuming $V_\text{total} = V_\ce{NaOH} + V_\ce{H2O}$ is a mistake. Used in chemical manufacturing, petroleum refining, cleaning compounds, drain cleaners. Office of Response and Restoration, Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. 2022, Specific Gravity of NaOH (& Formula, Definition, Infographic) rev.
The value of x is? 2CH 0 The specific gravity of a 50 % aqueous solution N a O H is 1.5298 g c m 3. WebSodium Hydroxide molecular weight. Soc. Corrosive to metals and tissue. To learn more, see our tips on writing great answers. Slowly absorbs carbon dioxide from the air to give solid products as crusts or precipitates.
USA.gov. $$, $$V = \frac{m(\ce{NaOH})}{\rho(\ce{NaOH})} + \frac{m(\ce{H2O})}{\rho(\ce{H2O})} = \pu{234.74 cm^3} + \pu{500 cm^3} = \pu{734.74 cm^3},\tag{2}$$, $$\rho_\text{sln} = \frac{\pu{1000 g}}{\pu{734.74 cm^3}} = \pu{1.361 g cm-3}.\tag{3}$$. Will not burn under typical fire conditions. The table was taken from "Perry's Chemical Engineers' Handbook" by Robert H. Perry, Don Green, Sixth Edition. The aggregate was in saturated surface dry condition with the specific gravity of 2.78 g/cm 3 and a maximum grain size of 2 mm was used for this experiment. 