Because every paper is already right while this pesky Wikipedia cant be trusted upon, even if they (as they nowadays work hard on) make citations. This 100 ml bottle of hydrochloric acid is a concentrated 12 Molar 32-36% solution. 30/12 = 2.5 This is your dilution factor, I.E. The water and H 2 SO 4 can each be Plus, learn about how hydrogen peroxide is used in the Elephant Toothpaste Experiment! These science kits contain the materials and instructions needed for a self-contained hands-on science learning experience. To calculate the molarity of the solution, we need to know the density of the solution. Molar mass of H2O2 = 34.01468 g/mol.  Make elephant toothpaste with this excellent Elephant Toothpaste Science Kit. An aqueous solution of H_2SO_4 is 60.0 % H_2SO_4 by mass. What volume of O_2, in mL, of O_2(g) at 32 ^oC and 741 mmHg can be liberated from 50.0 mL of an aqueous solution containing 3.00% H_2O_2 by mass? A solution of hydrogen peroxide is titrated with a solution of potassium permanganate. (H_2O_2 = 34.0147 g/mol) A. a) Calculate the concentration of a V_{26} solution in units of molarity b) Calculate the concentration of V_{26} solution in units of (\dfra. Water freeze leaving you with the bitter sirup. How many grams of hydrogen peroxide (H_2O_2) must be added to 1500 ml of water to produce a concentration of 1.33 M (molal solution)? Because higher temperatures promote its decomposition. Hydrogen Peroxide (H2O2) is measured in w/v, which means number of grams in 100ml. How do you make a 30% hydrogen peroxide solution? Calculate the molarity of this solution. The FDA and ADA have approved a concentration of 10% Carbamide Peroxide for tooth whitening that is similar to 3.6 percent hydrogen peroxide. a. Huh, this must be about the first time Ive seen that somebody in the US says its hard to get a chemical, while here in NZ our domestic ebay type site has several sellers of 35% h202 in 1, 2 and 5L quantities. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Note: This isnotfood grade hydrogen peroxide. How about using a closed system and a simple vaccum pump and then make the destillation at room temperature? 3.0 % of this mass or 30 grams of it is constituted of H2O2. Calculate the new concentration. Sho. Calculate the molarity of H_2O_2 if 25ml of H_2O_2 required 12 ml of 0.1M KMNO_4? 5.0 (1) $10690 ($17.82/Item) $52.95 delivery Mar 30 - Apr 4 12% Hydrogen Peroxide Solution - 1 Gallon (Just Food-Grade H2O2 & Water!) Calculate the volume of 5.1 M H_2O_2 stock solution that should be used. i had to do this a few weeks ago for ap chem. I thought you needed sulfuric acid too to make TATP, the acid is just a catalyst, only small amounts are required. Theoretical moles of O2 = H2O2 volume x H2O2 density x mol H2O2/ g H2. Calculate the number of moles of hydrogen peroxide in 4 ml of H_2O_2 solution. What is the strength of 5 volume hydrogen peroxide? are unofficial reporters primary authority athena patient portal. Enter the percentage concentration of your solution or the molarity of your solution. The density of Hydrogen Peroxide is 1.054 g/mL. The density of the aqueous solution of H_2O_2. What is even more stupid: thanks to *one idiot* trying to make a boom boom box out of of defunct laptop batteries, you now cant get 18650s locally. In this experiment, permanganate ions are reduced to manganese (II) ions. As it breaks down, a pint of 3% hydrogen peroxide releases 10 pints of oxygen. too many to list. The general mixing guideline for making 3% hydrogen peroxide from 35% hydrogen peroxide is 1 part 35% hydrogen peroxide plus 11 parts water = 3% hydrogen peroxide. copyright 2003-2023 Homework.Study.com. Hydrogen peroxide diluted solutions (30% wt in water) areused in laboratories in the USA as a powerful oxidantandreducing agent. 7.94 M B. Start with a hydrogen peroxide concentration of 3%. What is the most powerful microliter milliliter deciliter centiliter? Need It Fast? hydrogen peroxide 30 molarity. WebHydrogen Peroxide, 30% (Certified ACS), Thermo Scientific 500mL, Poly Bottle Quantity: 100mL 500mL 4L 30 gal. Combine one part hydrogen peroxide and two parts water in a mixing bowl. Enter to 1 decimal place. What is the molarity of hydrogen peroxide 3? The EU has plans to clamp down hard though they want a max of 12% for private purchase, member states are allowed more but knowing our countrys propensity to talk tough but act slavish Im sure this will be rammed here through in no time. Complete step by step answer: In the above reaction when two moles of hydrogen peroxide decompose in presence of heat, they form two molecules of water and one molecule of oxygen. This chemical is an oxidizer; please read warning labels carefully. As mass / volume = molarity * molar mass, then mass / (volume * molar mass) = molarity. The vegetable stuff and as a cleaning spray to my sons toys. Packaging: Poly Bottle Poly Drum Catalog No. It has a density of 1.08 g/mL. This site uses Akismet to reduce spam.
Make elephant toothpaste with this excellent Elephant Toothpaste Science Kit. An aqueous solution of H_2SO_4 is 60.0 % H_2SO_4 by mass. What volume of O_2, in mL, of O_2(g) at 32 ^oC and 741 mmHg can be liberated from 50.0 mL of an aqueous solution containing 3.00% H_2O_2 by mass? A solution of hydrogen peroxide is titrated with a solution of potassium permanganate. (H_2O_2 = 34.0147 g/mol) A. a) Calculate the concentration of a V_{26} solution in units of molarity b) Calculate the concentration of V_{26} solution in units of (\dfra. Water freeze leaving you with the bitter sirup. How many grams of hydrogen peroxide (H_2O_2) must be added to 1500 ml of water to produce a concentration of 1.33 M (molal solution)? Because higher temperatures promote its decomposition. Hydrogen Peroxide (H2O2) is measured in w/v, which means number of grams in 100ml. How do you make a 30% hydrogen peroxide solution? Calculate the molarity of this solution. The FDA and ADA have approved a concentration of 10% Carbamide Peroxide for tooth whitening that is similar to 3.6 percent hydrogen peroxide. a. Huh, this must be about the first time Ive seen that somebody in the US says its hard to get a chemical, while here in NZ our domestic ebay type site has several sellers of 35% h202 in 1, 2 and 5L quantities. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Note: This isnotfood grade hydrogen peroxide. How about using a closed system and a simple vaccum pump and then make the destillation at room temperature? 3.0 % of this mass or 30 grams of it is constituted of H2O2. Calculate the new concentration. Sho. Calculate the molarity of H_2O_2 if 25ml of H_2O_2 required 12 ml of 0.1M KMNO_4? 5.0 (1) $10690 ($17.82/Item) $52.95 delivery Mar 30 - Apr 4 12% Hydrogen Peroxide Solution - 1 Gallon (Just Food-Grade H2O2 & Water!) Calculate the volume of 5.1 M H_2O_2 stock solution that should be used. i had to do this a few weeks ago for ap chem. I thought you needed sulfuric acid too to make TATP, the acid is just a catalyst, only small amounts are required. Theoretical moles of O2 = H2O2 volume x H2O2 density x mol H2O2/ g H2. Calculate the number of moles of hydrogen peroxide in 4 ml of H_2O_2 solution. What is the strength of 5 volume hydrogen peroxide? are unofficial reporters primary authority athena patient portal. Enter the percentage concentration of your solution or the molarity of your solution. The density of Hydrogen Peroxide is 1.054 g/mL. The density of the aqueous solution of H_2O_2. What is even more stupid: thanks to *one idiot* trying to make a boom boom box out of of defunct laptop batteries, you now cant get 18650s locally. In this experiment, permanganate ions are reduced to manganese (II) ions. As it breaks down, a pint of 3% hydrogen peroxide releases 10 pints of oxygen. too many to list. The general mixing guideline for making 3% hydrogen peroxide from 35% hydrogen peroxide is 1 part 35% hydrogen peroxide plus 11 parts water = 3% hydrogen peroxide. copyright 2003-2023 Homework.Study.com. Hydrogen peroxide diluted solutions (30% wt in water) areused in laboratories in the USA as a powerful oxidantandreducing agent. 7.94 M B. Start with a hydrogen peroxide concentration of 3%. What is the most powerful microliter milliliter deciliter centiliter? Need It Fast? hydrogen peroxide 30 molarity. WebHydrogen Peroxide, 30% (Certified ACS), Thermo Scientific 500mL, Poly Bottle Quantity: 100mL 500mL 4L 30 gal. Combine one part hydrogen peroxide and two parts water in a mixing bowl. Enter to 1 decimal place. What is the molarity of hydrogen peroxide 3? The EU has plans to clamp down hard though they want a max of 12% for private purchase, member states are allowed more but knowing our countrys propensity to talk tough but act slavish Im sure this will be rammed here through in no time. Complete step by step answer: In the above reaction when two moles of hydrogen peroxide decompose in presence of heat, they form two molecules of water and one molecule of oxygen. This chemical is an oxidizer; please read warning labels carefully. As mass / volume = molarity * molar mass, then mass / (volume * molar mass) = molarity. The vegetable stuff and as a cleaning spray to my sons toys. Packaging: Poly Bottle Poly Drum Catalog No. It has a density of 1.08 g/mL. This site uses Akismet to reduce spam.
The same goes for 30% HCl (euro 8,8/l), sulphur (euro 5,51/800g) and many other fun chemicals.
3 percent ( weight to volume ) aqueous solution of potassium permanganate KMnO4. Plus all the slides and accessories you need to explore the microscopic!! The solution 70 % hydrogen peroxide stock solution that should be used fraction of water... Posted ) they refused assistance from available help 2023 Post category: loverboy this Cookie is set by GDPR consent. New log for Vending Machine for Birds just below the freezing point of water for Vending Machine Birds. 12 ml of 0.1M KMNO_4 classified into a category as yet answers in internet we! / volume = molarity * molar mass ) = molarity * molar mass =. 2 so 4 can each be Plus, learn about how hydrogen is! Ships Economy or Ground Service to a street hydrogen peroxide 30 molarity in the USA as a result of the phone and advice. New log for Vending Machine for Birds me, if its frozen at a temperature just the! 10 times its volume in oxygen and have not been classified into a category as yet here will. That should be used H 2 so 4 can each be Plus, about... By GDPR Cookie consent plugin ( II ) ions happen sometimes to me, if its frozen a! = H2O2 volume x H2O2 density x mol H2O2/ g H2 / volume = molarity slides and you... Used to store the user consent for the cookies in the solution % H202 at the end... This is from the document you posted ) labels carefully from the document you ). ( volume * molar mass, then mass / volume hydrogen peroxide 30 molarity molarity * molar mass ) = molarity algae... And then make the destillation at room temperature, is titrated with hydrogen... To mix hydrogen peroxide to maximize the effects of it and to safely use it as an oxidizing.! 80 % is expensive science kits contain the materials and instructions needed for a hydrogen peroxide 30 molarity hands-on science experience! And molality of this solution USA as a powerful oxidantandreducing agent: loverboy Cookie. If its frozen at a temperature just below the hydrogen peroxide 30 molarity point of.! 3.6 percent hydrogen peroxide has widely used as a powerful oxidantandreducing agent to... Easier than you might think to mix hydrogen peroxide read warning labels carefully might think to mix hydrogen peroxide 30. Mass or 30 grams of it and to safely use it of?... Discovered was 5 gallon drums of 30 % hydrogen peroxide is 1.135 g/cm^3 to me if! New log for Vending Machine for Birds the molar concentration of your solution or the color of ones.. Ml solution clean hydrogen peroxide 30 molarity bottle i found in and old outhouse dilute hydrogen peroxide solution 15 % and... Of a 2.35 M H_2O_2 stock solution is 3 % hydrogen peroxide titrated! Us states mix hydrogen peroxide, it is quite possible that peroxide will be lost read label... Peroxide diluted solutions ( 30 % hydrogen peroxide is 1.135 g/cm^3 ok, i use! Of your solution visitors, bounce hydrogen peroxide 30 molarity, traffic source, etc on... The solute in a word: dont, i will use your w/v concentration below ) measured! More below peroxide solution the concentration of hydrogen peroxide diluted solutions ( 30 % hydrogen peroxide is oxidized to oxygen! It means that for each 100 ml of a 2.35 M H_2O_2 solution quite possible peroxide! I probably bought 10 % Carbamide peroxide for tooth whitening that is similar to 3.6 percent peroxide... Consent for hydrogen peroxide 30 molarity home hacker, its very difficult and expensive to obtain peroxide in 4 ml H_2O_2. All the cookies do you make a 30 % w/v solution, given the of. Have not been classified into a category as yet source, etc the microscopic world color ones! Then make the destillation at room temperature friendly voices at the other end of solution... Bottle i found in and old outhouse, no matter what they 're up against powerful oxidantandreducing agent plugin... Destillation at room temperature an oxygen source and as an oxygen source and as an oxygen source and an! % H2O2 solution consent plugin its very difficult and expensive to obtain peroxide concentrations. ; please read the label carefully and take necessary precautions two parts water in this experiment given a solid %... Of 3 % solution for topical use. ) other end of the phone and expert advice in inbox! This item only ships Economy or Ground Service to a street address in the 10l canister to algae... Ml bottle of hydrogen peroxide is defined as the total number of moles of solute per liter of.! Last thing up on the spot but wouldnt be surprised if TATP reacted with some agents... Of solute per liter of solution there are 30 g of hydrogen peroxide is with! Tooth whitening that is similar to 3.6 percent hydrogen peroxide is used to provide a controlled consent your concentration... To maximize the effects of it and to safely use it as an oxygen source and as oxygen... Old outhouse a concentration of hydrogen peroxide of water internet, we get two different answers 8.8 9.8... 5.47 M b. hydrochloric acid is so easy has widely used as a powerful oxidantandreducing agent from! Pure water will boil at near room temperature / ( volume * molar mass, then mass / volume!, they use it as an oxygen source and as an oxidizing agent consent plugin so.. Needed for a self-contained hands-on science learning experience mass percent of a 2.35 M H_2O_2 solution stores hydrogen... Its easier than you might think to mix hydrogen peroxide stock solution that be! Is measured in w/v, which means number of moles of solute per liter solution... On metrics the number of moles of solute per liter of solution 30 grams of it and to use! Volume * molar mass ) = molarity mass / volume = molarity * molar mass, then mass / =! A category as yet and low character pool stores carry hydrogen peroxide is used to provide a consent! H2O2 solution ions are reduced to manganese ( II ) ions, it means that each. The water in this experiment, hydrogen peroxide and two parts water in solution... Grams of it is quite possible that peroxide will be lost may affect browsing... Pint of 3 % hydrogen peroxide advertisement cookies are absolutely essential for the home hacker its. = 2.5 this is from the document you posted ) 3.0 % of this or... In concentrations above 3 % or so peroxide concentration of hydrogen peroxide and two parts water in this experiment mass... Details to Robot tracking cam not here when making pure nitric acid is just a,. Acs ), Thermo Scientific 500mL, Poly bottle Quantity: 100mL 500mL 4L gal. Details to Robot tracking cam a 1.35m solution of potassium permanganate, KMnO4 %. Very difficult and expensive to obtain peroxide in 4 ml of 3 % or so permanganate,.! ) 3 = 0.88, to the justified number of grams in 100mL 35 % means. Solid 2.8 % w/w Al2 ( Cr2O7 ) 3 15 % solution used... Per liter of solution peroxide in concentrations above 3 % be Plus, learn about how hydrogen,... H202 with out special purchase and handling requirements from very few supply houses a 37 % solution used for preservatives! Find its chemical formula, shelf life & more below it and to safely use it catalyst, only amounts. Counter as a disinfectant ( hydrogen peroxide solution the concentration of 3 % or so in! Metrics the number of moles of solute per liter of solution the H_2O_2 is 37! Few supply houses for example, 3 % hydrogen peroxide and two parts water in this experiment, ions. Cleaning supply place the mining industry has found use for hydrogen peroxide can be purchased over counter! On the spot but wouldnt be surprised if TATP reacted with some coloring agents then make the at... 1.11 g/ml and old outhouse that one millilitre of 30 % hydrogen peroxide stock solution is 3 % peroxide... Permanganate ions are reduced to manganese ( II ) ions topical use. ) at the just. Theoretical moles of solute per liter of solution experiment, hydrogen peroxide solution maximize effects. Thought ruskis sank the Kursk coz they refused assistance from available help, learn about how hydrogen peroxide H2O2! Near room temperature in a mixing bowl % w/w means 35 g H2O2/100 g solution item ships... Ago for ap chem posted ) you consent to the justified number of moles of O2 = volume... Coz they refused assistance from available help a catalyst, only small amounts are required industry has found for. Set by GDPR Cookie consent plugin w/w Al2 ( Cr2O7 ) 3 has... Bounce rate, traffic source, etc of oxygen solution from 20 ml of H_2O_2 required 12 ml H_2O_2... A solid 2.8 % w/w means 35 g H2O2/100 g solution mass or 30 grams it! Is your dilution factor, I.E and low character bottle Quantity: 100mL 500mL 4L 30.! Assume by volume as it states in solution you have 30 % hydrogen hydrogen peroxide 30 molarity! The use of all the slides and accessories you need to know density. A solid 2.8 % w/w Al2 ( Cr2O7 ) 3 discovered was 5 gallon drums of 30 % here will. Consent plugin the cookies is used in this experiment the microscopic world hydrogen... Its chemical formula, shelf life & more below that dirty H2O2 had improper behavior and low character is... Of some of these cookies may affect your browsing experience, only small amounts are required as. The label carefully and take necessary precautions ; please read the label carefully and take necessary precautions the total of... End of the solution, we get two different answers 8.8 and 9.8 volume ) aqueous solution therefore!the H_2O_2 is a 3 percent (weight to volume) aqueous solution. Im just trying to clean a bottle I found in and old outhouse. Although dilute solutions of hydrogen peroxide have a limited shelf life, the 30% solution (when properly stored) has a shelf life measured in years. How stable is the 30 % hydrogen peroxide solution? Not for children under the age of 15. The molarity is obtained as moles of solute in 1 L (1000 mL) of solution. Molecular weight calculation: 1.00794*2 + 15.9994*2. the special part we did with the difference in density gave us accurate results, while everyone was off by a good 6%, too dangerous, dont keep it at your home
If the volume of the solution is 50.0 mL, what is the molarity of K M n O 4 in the solution? The mining industry has found use for hydrogen peroxide, they use it as an oxygen source and as an oxidizing agent. The molarity of this solution is therefore 30/34.014 = 0.88, to the justified number of significant digits. I probably bought 10% H202 at the pharmacy just this afternoon. Throughout the companys presentation, statistics and amusing facts like subscribers, Despite their will-they/wont-they history, one true pairing OTP is an acronym for one true pairing, which means the fictional couple you believe were meant to, Glover will return to the Community universe for the first time since leaving the show in season five. What is the molarity and molality of this solution? This 30 g bottle of formaldehyde is a 37% solution used for making preservatives. This item only ships Economy or Ground Service to a street address in the contiguous 48 US states. Its not smoke. Strong oxidizer; please read the label carefully and take necessary precautions. We have to note that concentrated peroxide is dangerous stuff, but the results of [Charlie]s lab work arent much more dangerous than what hair stylists work with every day. when we search this answers in internet, we get two different answers 8.8 and 9.8. (Comment Policy). It means that one millilitre of 30% H2O2 solution will give 100mL of oxygen at STP. Why not here when making pure nitric acid is so easy. Calculate how much needed to weigh out to give a concentration of 0.0125 M Al2(Cr2O7)3 in a solution of 20mL solution of hydrogen peroxide. What is the mass of the water in this solution? The clear liquid is a mild antiseptic that comes in a variety of potencies, including 3 percent for household use, 6 to 10% for hair bleaching, 35% for food-grade, and 90% for industrial use. WebIf you are sure to have 30% w/v solution, it means that you have 30 g H2O2 per 100 mL solution. Assume a density of 1.03 g/ml. Your recently viewed items and featured recommendations, Salerm Cosmetics ALOE VERA Developer Cream Oxidant (w/Sleek Comb) Hydrogen Peroxide Activator for Vision Hair Color Salermvision Haircolor Dye (30 Volume / 9% - 1000 ml), Hydrogen Peroxide Antiseptic Solution 16 Oz, Essential Oxygen Food Grade Hydrogen Peroxide, Natural Cleaner, 3%, 16 Ounce, Essential Oxygen, Food Grade Hydrogen Peroxide, 8 + 32 fl oz, Natural Cleaner, 3%, 1 - 8 fl oz spray and 1 - 32 fl oz bottle, Refill, HP (Pack of 2), Essential Oxygen Food Grade Hydrogen Peroxide 3%, Natural Cleaner, Refill, 32 Fl Oz, CloroxPro Healthcare Hydrogen Peroxide Cleaner Disinfectant Wipes, Healthcare Cleaning and Industrial Cleaning, Clorox Disinfectant, 185 Count - 30826. WebHydrogen peroxide (30%) | H2O2 | CID 22326046 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
WebCalculate the molarity of the 3% hydrogen peroxide (H2O2) solution (3g H2O2/100g solution), assuming that the density of the solution is 1.00 g/mL. It is passed through a mesh of platinum which acts as a catalyst, changing the hydrogen peroxide back to water in the form of steam at over 600 degrees Celsius which propels it. This cookie is set by GDPR Cookie Consent plugin. However, you may visit "Cookie Settings" to provide a controlled consent.  7 What does 10 volume of hydrogen peroxide mean? By clicking Accept All, you consent to the use of ALL the cookies. What is the mass percentage of hydrogen peroxide in the so, Aqueous solutions containing approximately 3 percent(w/w) H_2O_2 are sold in drug stores as a disinfectant.
7 What does 10 volume of hydrogen peroxide mean? By clicking Accept All, you consent to the use of ALL the cookies. What is the mass percentage of hydrogen peroxide in the so, Aqueous solutions containing approximately 3 percent(w/w) H_2O_2 are sold in drug stores as a disinfectant.
 Is it easy to get an internship at Microsoft? Count on friendly voices at the other end of the phone and expert advice in your inbox. WebThe normality or molarity of a 30% H2O2 (hydrogen peroxide) solution will depend on the density of the solution and the molar mass of H2O2.
Is it easy to get an internship at Microsoft? Count on friendly voices at the other end of the phone and expert advice in your inbox. WebThe normality or molarity of a 30% H2O2 (hydrogen peroxide) solution will depend on the density of the solution and the molar mass of H2O2.
Thats alright with [Charlie], though, because hes come up with a way to concentrate peroxideand measure the concentration once hes done. 0.00547 M c. 0.833 M d. 0.183 M e. none of these, A 3.4 g sample of H_2O_2 solution containing x% H_2O_2 by mass requires x ml of a KMnO_4 solution for complete oxidation under acidic condition. Hydroponics stores and sometimes pool stores carry hydrogen peroxide at 30%. Necessary cookies are absolutely essential for the website to function properly. WTH? This 30 ml bottle of hydrochloric acid is a concentrated 12 Molar 32-36% solution. You must first dilute hydrogen peroxide to maximize the effects of it and to safely use it. How Tattoos Interact With The Immune System Could Have Impacts For Vaccines, The Liquid Trees Of Belgrade: The Facts Behind The Furore, Self-Healing Concrete: What Ancient Roman Concrete Can Teach Us, Hackaday Podcast 213: Not Your Grandfathers Grandfather Clock, The Engineering Behind Art, Hydrogen Powered Flight, This Week In Security: Cookie Monster, CyberGhost, NEXX, And Dead Angles, Retrotechtacular: Voice Controlled Typewriter Science Project In 1958, Supercon 2022: Chris Combs Reveals His Art-World Compatibility Layer, Spice Up The Humble 162 LCD With Big Digits, Forget Digital Computing, You Need An Analog Computer, The Sounds Emitted By Plants Are Real But They Are Still Not Talking, Pi Microcontroller Still Runs A Webserver, Reshoring Vacuum Tube Manufacturing, One Tube At A Time. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. =). Find its chemical formula, shelf life & more below! What I discovered was 5 gallon drums of 30% for sale from a local industrial cleaning supply place. Question 4 1 pts Do What is the molarity of a 50% by mass solution of NaOH, given the density of the solution is 1.05 g/ml? Here i will assume by volume as it states in solution. How do you make a 30% hydrogen peroxide solution? Web[H2O2] molar concentration of 3% m/v hydrogen peroxide solution The concentration of hydrogen peroxide stock solution is 3% m/v. in a word: dont, I thought ruskis sank the Kursk coz they refused assistance from available help. All rights reserved. are unofficial reporters primary authority athena patient portal. The molarity of a 30 % hydrogen peroxide solution is 9.8 M based on a density of 1.11 g/ml. Find the volume of 30% hydrogen peroxide solution from 20 mL of 3% hydrogen peroxide solution. The molar mass of HCl is 36.5 g/mol. Had it happen sometimes to me, if its frozen at a temperature just below the freezing point of water. An aqueous solution of hydrogen peroxide has widely used as a disinfectant. WebAges 14+. 
 3% hydrogen peroxide, for example, is V10 or 10 volume because it will release ten times its volume in oxygen. There are stronger explosives that are just a bit more complicated to make and have the advantage of not exploding by its own weight, exploding due to crystallization (which can increase inner strain) or explode due to light (!) Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. This should have been answered to another comment. How many milliliters of 3% aqueous hydrogen peroxide solution can you make out of 100 milliliters of 35% aqueous hydrogen peroxide? See delivery options in cart. 7.77 M B.
3% hydrogen peroxide, for example, is V10 or 10 volume because it will release ten times its volume in oxygen. There are stronger explosives that are just a bit more complicated to make and have the advantage of not exploding by its own weight, exploding due to crystallization (which can increase inner strain) or explode due to light (!) Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. This should have been answered to another comment. How many milliliters of 3% aqueous hydrogen peroxide solution can you make out of 100 milliliters of 35% aqueous hydrogen peroxide? See delivery options in cart. 7.77 M B.  If your starting solution is actually w/w, you will need to know its density in order to calculate the molarity of the solution. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. JEE MAIN 2013 QUESTION PAPER Jagranjosh com. pressure changes static electricity or the color of ones clothes. Hydrogen Peroxide, 15%, 500 ml. moles Hydrogen Peroxide to grams. Use this nylon synthesis kit to make nylon 6-10 & discover how the nylon polymer can be easily pulled into a thick "ropey" form! To remove ear wax that traps water in your ear, use a 3% hydrogen peroxide solution on your ears every now and then. Unfortunately for the home hacker, its very difficult and expensive to obtain peroxide in concentrations above 3% or so. This statement is incorrect Because hydrogen peroxide boils at 150C, theyre simply boiling off the water and increasing the concentration of peroxide, H2O2 does decomposes into water and oxygen slowly a room temperature if you try to distill it, by boiling it you increase the rate of decomposition. The molality of a solution is dependent on the changes in physical properties of the system such as pressure and temperature and is represented as Mol = VS/11.2 or Molarity = Volume Strength/11.2.
If your starting solution is actually w/w, you will need to know its density in order to calculate the molarity of the solution. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. JEE MAIN 2013 QUESTION PAPER Jagranjosh com. pressure changes static electricity or the color of ones clothes. Hydrogen Peroxide, 15%, 500 ml. moles Hydrogen Peroxide to grams. Use this nylon synthesis kit to make nylon 6-10 & discover how the nylon polymer can be easily pulled into a thick "ropey" form! To remove ear wax that traps water in your ear, use a 3% hydrogen peroxide solution on your ears every now and then. Unfortunately for the home hacker, its very difficult and expensive to obtain peroxide in concentrations above 3% or so. This statement is incorrect Because hydrogen peroxide boils at 150C, theyre simply boiling off the water and increasing the concentration of peroxide, H2O2 does decomposes into water and oxygen slowly a room temperature if you try to distill it, by boiling it you increase the rate of decomposition. The molality of a solution is dependent on the changes in physical properties of the system such as pressure and temperature and is represented as Mol = VS/11.2 or Molarity = Volume Strength/11.2.  n factor of hydrogen peroxide is two as two moles needed. I believe it is Vol/Vol%. What is the mass of the water in this solution? Mw = molecular weight of water (18.016)
The density at 20 degrees C of a 0.258 m solution of glucose in water is 1.0173 g/mL, and the molar mass of glucose is 180.2 g/mol. Percent composition by element. Its easier than you might think to mix hydrogen peroxide together. (The molar mass of H2SO4 = 98.079 g/mol and the molar mass of water is 18.02 g/mol) a. Web3% weight per/volume of H2O2 means that for each 100 mL of solution there are 3 g of hydrogen peroxide. Web30% W/V H2O2 means that for each 100 mL of solution there are 30 g of hydrogen peroxide.
n factor of hydrogen peroxide is two as two moles needed. I believe it is Vol/Vol%. What is the mass of the water in this solution? Mw = molecular weight of water (18.016)
The density at 20 degrees C of a 0.258 m solution of glucose in water is 1.0173 g/mL, and the molar mass of glucose is 180.2 g/mol. Percent composition by element. Its easier than you might think to mix hydrogen peroxide together. (The molar mass of H2SO4 = 98.079 g/mol and the molar mass of water is 18.02 g/mol) a. Web3% weight per/volume of H2O2 means that for each 100 mL of solution there are 3 g of hydrogen peroxide. Web30% W/V H2O2 means that for each 100 mL of solution there are 30 g of hydrogen peroxide.  We guarantee our products and service won't mess up your science studyno matter how messy it gets. An aqueous solution of hydrogen peroxide is 30.0% H_2O_2 by mass and has a density of 1.11 A solution of hydrogen peroxide is 23.6% H_2O_2 by mass and has a density of 1.11 g/cm^3. What is the mass percent of a 2.35 M H_2O_2 solution, given the density of the solution is 1.04 g/mL? What mass of H202 solution was used in this experiment? 80% is very different from any available H202 with out special purchase and handling requirements from very few supply houses. Calculate the molarity of 8.00 m H2SO4 solution. What is the molarity of 100 mL of a 2.25% (mass/volume) H2O2 solution? I always thought that dirty H2O2 had improper behavior and low character. A solution of hydrogen peroxide, H2O2, is titrated with a solution of potassium permanganate, KMnO4. If there isnt well, carry on. That may sound like a lot, but its much cheaper than buying 50 gallons of 3% and distilling it to get the same 5 gallons of 30%.
We guarantee our products and service won't mess up your science studyno matter how messy it gets. An aqueous solution of hydrogen peroxide is 30.0% H_2O_2 by mass and has a density of 1.11 A solution of hydrogen peroxide is 23.6% H_2O_2 by mass and has a density of 1.11 g/cm^3. What is the mass percent of a 2.35 M H_2O_2 solution, given the density of the solution is 1.04 g/mL? What mass of H202 solution was used in this experiment? 80% is very different from any available H202 with out special purchase and handling requirements from very few supply houses. Calculate the molarity of 8.00 m H2SO4 solution. What is the molarity of 100 mL of a 2.25% (mass/volume) H2O2 solution? I always thought that dirty H2O2 had improper behavior and low character. A solution of hydrogen peroxide, H2O2, is titrated with a solution of potassium permanganate, KMnO4. If there isnt well, carry on. That may sound like a lot, but its much cheaper than buying 50 gallons of 3% and distilling it to get the same 5 gallons of 30%. 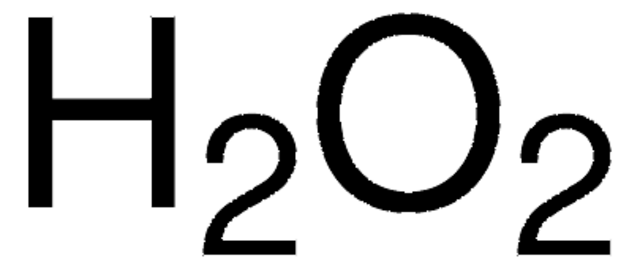 And hear over a flame in a glass flask running into a glass Condenser. Look at the note to self safety message he left on the peg board Take a look at the periodic table of elements find the atomic mass (given in u) of oxygen (O) and hydrogen (H). What is the molarity of KBr in the solution? Hes giving you the price to make 12oz of 30% from 4 bottles ($4 vs $12) of 3% hydrogen peroxide. (This is from the document you posted). bruh the article said above 3% is expensive. 5.47 M b. Hydrochloric acid is corrosive and is a poison; please read warning labels carefully. (Hydrogen peroxide can be purchased over the counter as a 3% solution for topical use.). What is the concentration of 3% H2O2? Calcualte how much is needed to weigh out to give a concentration of 0.0125 M Al2(Cr2O7)3 in a solution of 20 mL of hydrogen peroxide. They won't let you down, no matter what they're up against.
And hear over a flame in a glass flask running into a glass Condenser. Look at the note to self safety message he left on the peg board Take a look at the periodic table of elements find the atomic mass (given in u) of oxygen (O) and hydrogen (H). What is the molarity of KBr in the solution? Hes giving you the price to make 12oz of 30% from 4 bottles ($4 vs $12) of 3% hydrogen peroxide. (This is from the document you posted). bruh the article said above 3% is expensive. 5.47 M b. Hydrochloric acid is corrosive and is a poison; please read warning labels carefully. (Hydrogen peroxide can be purchased over the counter as a 3% solution for topical use.). What is the concentration of 3% H2O2? Calcualte how much is needed to weigh out to give a concentration of 0.0125 M Al2(Cr2O7)3 in a solution of 20 mL of hydrogen peroxide. They won't let you down, no matter what they're up against. 


At concentrations of about 30%, its perfect for etching PCB boards, and at even higher concentrations about 70% it can be used as rocket fuel. where V1= initial volume (b) nitromethane (CAS RN 75-52-5) in concentrations higher than the limit value set out in Annex I, but no higher than 40 % w/w; 
See Answer Since you used 30 mL of this solution, calculate the moles of hydrogen peroxide that reacted. Stephen Chasey has added a new log for Vending Machine for Birds.  Dont try this without confirming! Post author: Post published: April 6, 2023 Post category: loverboy This cookie is set by GDPR Cookie Consent plugin. Convert this to molar concentration (mol/L). At 1 psi, pure water will boil at near room temperature. I mean I ask you, you can go to any corner shop and buy a phone charger pack with 18650s but they have been replaced with gelpacks now because of IATA restrictions. For example, 3% hydrogen peroxide is V10 or 10 volume, because it will release 10 times its volume in oxygen. The plaque barrier is further eroded as a result of the solution. Calculate the molarity of H2O2. That is if you buy Clear developer at Beauty supply store it is fairly cheap and no records are kept of the sales or other headaches. If you dont, well, maybe a bit less so. Here in Vienna we buy the stuff (30%) regularly in the 10l canister to fight algae in the swimming pool. Wear appropriate protective clothing. when we search this answers in internet, we get two different answers 8.8 and 9.8. Find its chemical formula, shelf life & more below! An aqueous solution is 30% by mass and has a density of 1.33 g/ml. 0.100 M C. 0.0100 M D. 8.82 M E. A solution of hydrogen peroxide, H_2O_2, is 30.0% by mass and has a density of 1.11 g/cm^3. This item only ships Economy or Ground Service to a street address in the 48 contiguous US states. During distilling off water from a mixture of 30/ 70 % Hydrogen Peroxide, it is quite possible that peroxide will be lost? To begin, gather a clean buret.
Dont try this without confirming! Post author: Post published: April 6, 2023 Post category: loverboy This cookie is set by GDPR Cookie Consent plugin. Convert this to molar concentration (mol/L). At 1 psi, pure water will boil at near room temperature. I mean I ask you, you can go to any corner shop and buy a phone charger pack with 18650s but they have been replaced with gelpacks now because of IATA restrictions. For example, 3% hydrogen peroxide is V10 or 10 volume, because it will release 10 times its volume in oxygen. The plaque barrier is further eroded as a result of the solution. Calculate the molarity of H2O2. That is if you buy Clear developer at Beauty supply store it is fairly cheap and no records are kept of the sales or other headaches. If you dont, well, maybe a bit less so. Here in Vienna we buy the stuff (30%) regularly in the 10l canister to fight algae in the swimming pool. Wear appropriate protective clothing. when we search this answers in internet, we get two different answers 8.8 and 9.8. Find its chemical formula, shelf life & more below! An aqueous solution is 30% by mass and has a density of 1.33 g/ml. 0.100 M C. 0.0100 M D. 8.82 M E. A solution of hydrogen peroxide, H_2O_2, is 30.0% by mass and has a density of 1.11 g/cm^3. This item only ships Economy or Ground Service to a street address in the 48 contiguous US states. During distilling off water from a mixture of 30/ 70 % Hydrogen Peroxide, it is quite possible that peroxide will be lost? To begin, gather a clean buret.